Swedish investigators set out to uncover the pathways that HHV-6B might utilize in triggering MTLE. They found that HHV-6B infection altered expression of MAPK genes, suggesting a possible pathogenic mechanism of HHV-6B in mesial temporal lobe epilepsy (MTLE).
An association between HHV-6B and mesial temporal lobe epilepsy (MTLE) was first described in 2000 upon examination of resected brain tissue (Uesugi 2000). Soon afterward, NINDS investigators found elevated HHV-6B viral loads in cells appearing to be astrocytes in brain tissue from patients with MTLE when compared to tissues from individuals with neocortical epilepsy (Donati 2003). Altered gene expression (MCP-1, glial fibrillary acidic protein, and NF-κB) has been found in the brains of those with HHV-6B and MTLE or mesial temporal sclerosis, which leads to MTLE, but not in control tissues (Kawamura 2015, Li 2011). However, the mechanisms behind HHV-6 involvement in MTLE have not been established.

Anna Fogdell-Hahn, PhD, Associate Professor
Department of Clinical Neuroscience
Karolinska University Hospital
A team of investigators led by Anna Fogdell-Hahn, PhD, compared gene expression in epileptic patient tissues, both positive and negative for HHV-6B infection. The group used RNA from the fresh frozen hippocampal tissues of 10 HHV-6B+ and 14 HHV-6B- epileptic patients and measured expression of over 40,000 transcripts. Expression of MAP2K4 and MAPK9 were found to be elevated (though not significantly) among HHV-6B-infected samples.
Exploring this finding further, the team infected Molt-3 T cells with HHV-6B and measured in vitro expression of genes in the MAPK pathway, again observing upregulation of MAP2K4 expression.
Upregulation of the corresponding protein, MKK4, was also found 6 days post-infection. Moreover, 26 (29%) of 90 other MAPK genes were differentially expressed at 3 days post-infection, with all but three of these genes revealing higher expression in infected cells compared to uninfected cells. Genes throughout the p38 MAPK and SAPK/NK signaling cascades, from beginning to end, were affected, and HHV-6B also induced JNK3 expression and expression of four DUSP genes that inactivate kinases. The HHV-6B-induced changes to gene expression did not appear to result from changes in DNA methylation. Together, the data suggested that HHV-6B was not only associated with altered expression and activity of the MAPK pathways, but was able to cause these changes.
Ingenuity Pathway Analysis software indicated that the altered gene expression may in turn affect networks involved with post-translational modification, cell death and survival, organismal injury and abnormalities, and cell morphology/organ morphology/skeletal and muscular system development and function. In epilepsy studies, a dysregulation of MAPK pathways has been noted (Hansen 2014, Salman 2017, Tai 2017) and would be a plausible mechanism explaining impaired uptake of neurotransmitters by astrocytes which might in turn give rise to epilepsy symptoms.
Although statistical significance was not reached in results from the brain tissue of patients with MTLE, the findings led the team to postulate that HHV-6B may contribute to MTLE by interfering with the vesicular transport system through the MAPK pathway, thereby impairing neurotransmitter uptake, and perhaps also by interfering with cell death pathways.
JNK3, c-MYC, and p53 expression were differentially expressed among infected cells compared to uninfected cells, and all three impact cell survival and proliferation. While p53 was over-expressed in the HHV-6B-infected cells, the virus is able to sequester p53 in the cytoplasm (ref), inhibiting its activity, so the upregulated p53 observed in the Molt-3 cells may have resulted from a feedback response of inhibited p53. Not only are cell death and cytoplasmic vacuolization pathways related, but mice without p53 have developed a more intense epileptic phenotype (Engel 2010).
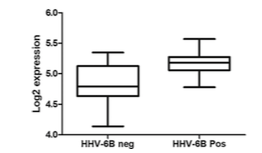
Expression of MAPK2k4 gene in MTLE hippocampus tissue positive or negative for HHV-6B DNA. Source: Virus Research
The MAPK pathways have also been found to be involved in cancer. The roles of HHV-6A and –B have been studied in the contexts of many types of cancer, but at present, the contribution of either virus to cancer development is unclear. Further examination of the HHV-6B-induced changes to expression of MAPK pathway genes involved in cell death and proliferation may be worthwhile avenues to explore in the setting of HHV-6-associated oncogenesis.
In understanding how HHV-6B can affect pathways associated with MTLE, the specific roles of the virus in epilepsy and other neurological disorders can be better evaluated, and targets for future treatments can be considered. Further characterization of HHV-6B-associated effects on the MAPK pathways, neurotransmitter uptake and vesicle transportation, and cell death might include the use of glial cell cultures and measurement of proteins associated with differentially expressed genes.
Find the full paper here: Engdahl 2018.

