Multiple studies have found evidence of reactivated HHV-6 (and, to a lesser degree, HHV-7) in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Valganciclovir, cidofovir and foscarnet inhibit replication of HHV-6 in vitro, and both ganciclovir and foscarnet are used to treat HHV-6 encephalitis in transplant patients. Acyclovir and valacyclovir, while effective for HSV1/2, are ineffective against HHV-6 and HHV-7. Ganciclovir has only very weak activity against HHV-7 (Chemaly 2019, DeClercq 2001).
However, a number of in vitro studies indicate that artesunate, an antimalarial drug derived from artemisinin, has anti-herpesvirus properties against multiple viruses including EBV, HHV-6, CMV, BK and JC virus (Milbradt 2009, Marschall 2011, Chemaly 2019).
A team from Ukraine identified 255 people with ME/CFS who had evidence of reactivated HHV-6 or HHV-7 infection and followed them with repeated viral studies for 3 months. In 192 of them, antiviral agents were administered continuously over three months: valacyclovir (68 patients), valganciclovir (64 patients) or artesunate (60 patients). In 63 patients, no antiviral was administered. The choice of treatment was left to individual clinicians, not randomized.
The investigators diagnosed viral reactivation by isolating leukocytes and identifying viral DNA using PCR. HHV-6 infection was diagnosed in 48% of cases and HHV-7 in 76% of cases, with 24% testing positive for both. It is important to note that HHV-6 and HHV-7 are generally not detectable in the plasma of ME/CFS patients, and clinical laboratory testing in the US and Japan is done almost exclusively on plasma samples. In Europe, whole blood testing or testing of peripheral blood mononuclear cells (PBMCs) is more common.
As shown in Figure 1, all three antiviral therapies produced complete eradication of detectable virus over time in a substantial fraction of patients, whereas detectable virus remained in the great majority of the untreated patients. Of the three drugs, artesunate produced the highest percentage of complete responses.
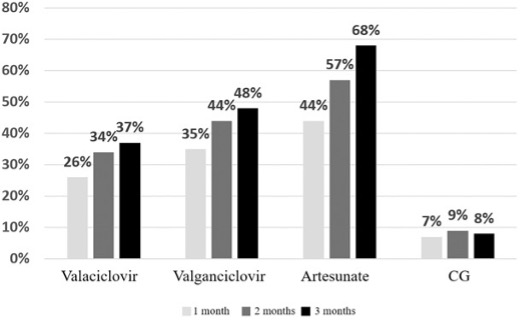
Figure – Relative proportion of complete viral eradication at 1, 2 and 3 months with each antiviral agent and in patients given no antivirals. [Valacyclovir is misspelled]
The authors did not assess whether symptoms or functional capacity improved with therapy: the clinical value of treatment was unaddressed. They did report, however, on apparent adverse effects of treatment. Among patients given valganciclovir or artesunate, mild neutropenia was reported in about 20%, increased concentration of liver enzymes in 15-20%, and mild anemia in about 5-7%.
Thus, it remains unclear, however, if reactivation of these viruses plays a causal role in generating the symptoms of ME/CFS, and whether successfully reducing viral load leads to improvement in those symptoms.
This report suggests the need for further controlled virologic and clinical studies of artesunate in diseases which are or may be caused by HHV-6 and HHV-7. It does not shed light on the value of these agents as treatments in ME/CFS.
Real the full article: Maltsev 2022

