The main risk factor for HHV-6 reactivation was low absolute lymphocyte count.
With all forms of allogeneic hematopoietic cell transplantation (HCT), HHV6 reactivation arises in 47% to 72% of patients, on average, posing a significant risk of morbidity and mortality. With umbilical cord blood transplant, it is even higher. Haploidentical hematopoietic cell transplantation (haplo HCT), typically from a family donor, has multiple advantages, when it can be done.
The frequency and consequences of HHV-6B reactivation after haploidentical hematopoietic cell transplantation (haplo HCT) with post-transplant cyclophosphamide (PT-Cy) has not been extensively studied.
A team from Saint-Antoine Hospital in Paris performed a retrospective study in 100 consecutive patients receiving haplo HCT with PT-Cy. HHV-6 reactivation and DNA loads were monitored weekly until day +100. GvHD prophylaxis consisted of cyclosporine A, mycophenolate mofetil (MMF), and PT-Cy for all patients. Infection prophylaxis consisted of amoxicillin, trimethoprim-sulfamethoxazole (or atovaquone), and valacyclovir (or acyclovir). Patients were systematically monitored for CMV and aspergillosis twice a week, and for HHV6, EBV, BK virus, and Toxoplasma gondii once a week until at least day +100. In some of the patients, antithymocyte globulin (ATG) was added for GVHD prophylaxis.
HHV6 real-time polymerase chain reaction (PCR) weekly during the first 100 days after haplo HCT. HHV6 reactivation was defined as the detection of at least 500 IU/mL HHV6 DNA in peripheral blood in two consecutive samples. In the event of HHV6 reactivation, HHV6 DNA loads were monitored until they became lower than 500 international units (IU) per mL. No individuals with iciHHV6 were included in the study.
Results:
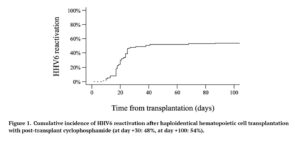
- Cumulative incidence of HHV6 reactivation was 54%, with a medium time of onset of 20 days post-transplantation, as shown in Figure 1;
- Clinically significant HHV6 infections were infrequent (7%), were associated with higher HHV6 DNA loads, and had favorable outcomes after antiviral therapy;
- Clinically significant HHV6 infections included colitis, encephalitis, hepatitis, myelosuppression with secondary graft failure, and pneumonitis;
- In cases of clinically significant HHV6 infection, the median highest HHV6 DNA load was 468,447 IU/mL (range, 10,400-1,658,173) compared to 6,135 IU/mL (range, 524-505,945) IU/mL in patients with no signs of infection (p < 0.001);
- The main risk factor for HHV6 reactivation was a low absolute lymphocyte count (ALC) < 290/μL on day +30;
- Even though combining ATG with PT-Cy can induce a significant reduction in early gamma-delta (γδ) T-cell proliferation, and is associated with a higher risk of increase in EBV viral loads, adding ATG to PT-Cy did not increase the incidence of HHV6 reactivation (52% with ATG versus 79% without ATG);
- CMV reactivation occurred about as often as HHV6 reactivation, and both viruses reactivated in 32% of patients.
- HHV-6 reactivation was associated with:
- A higher incidence of grade II-IV GvHD (39% vs 9%), a difference that just missed being statistically significant with multivariable analysis to reduce the impact of potential confounders);
- Poorer platelet recovery (HR 1.81);
- Similar rate of lymphocyte reconstitution;
- Similar rate of neutrophil engraftment at day +30;
- Similar incidence of relapse;
- Similar overall survival and non-relapse mortality.
There was also an association between higher HHV-6 DNA load and the development of grade II-IV GvHD (p=0.02).
The authors conclude that combining ATG and PT-Cy in people with haplo HCT is safe in terms of the risk of HHV6 reactivation and infection. They also conclude that weekly monitoring of HHV-6B reactivation is not necessary in these patients, although patients with a low ALC on day +30 face a higher risk of HHV6 reactivation and may require careful monitoring.
Read the full article: Paviglianiti 2024 (doi:10.46989/001c.92525) Published online. No PubMed link yet

