Results from preclinical and Phase 2 studies were encouraging, but Phase III trials were cancelled
Therapeutic advances, such as improved treatment of graft-versus-host disease, have improved the success of hematopoietic cell transplantation (HCT) from HLA-mismatched donors. Unfortunately, the high degree of immunosuppression required for success leads to an increased risk of potentially severe opportunistic infections from various double-stranded DNA viruses, including HHV-6, adenovirus (AdV), BK virus (BKV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), and JC virus (JCV). There are approved antivirals to treat CMV, but not the other viruses, and the antivirals used to treat CMV have significant toxicities.
Since the opportunistic infections are due largely to impaired cellular immunity, it has been theoretically attractive to try to develop cell therapy, as described in two recent publications.
A multi-institutional team, with commercial support, developed a partially-HLA-matched, allogeneic, “off-the-shelf”, virus-specific memory T cell (VST) product that specifically targets multiple epitopes on each of the above-mentioned viruses. The product, originally called Viralym-M and then ALVR105, was renamed posoleucel before Phase III trials were begun.
This mix of cells was obtained from multiple healthy seropositive individuals, and then expanded by a single round of in vitro stimulation with a mix of antigens from the targeted viruses. The resultant VSTs comprise activated CD8+ and CD4+ T cells expressing a broad repertoire of antiviral TCRs. Specifically, the mix was 64.7% CD4+, 31.5% CD8+ and 0.2% NK cells.
A recently published report (Vasileiou 2024) demonstrates that upon exposure to target viruses, posoleucel cells execute an array of effector functions including:
- Upregulation of molecules on the T cell surface known to be important in mediating cellular immunity and inducing humoral immunity (e.g., CD40L, CD69, 4-1BB)
- Production of Th1-polarized cytokines and related effector molecules known to have direct and indirect antiviral properties (e.g., GrB, IFN-γ, IL-2, TNF-α, and GM-CSF)
- Antigen-specific cytolytic activity, demonstrated by a challenge of posoleucel cells to at least two antigens from each of the six viruses
- Negligible alloreactive potential, as measured using HLA-mismatched cells as targets.
The evidence that exposure of posoleucel cells to viral antigens elicited a Th1 response is shown in Figure 1, below.
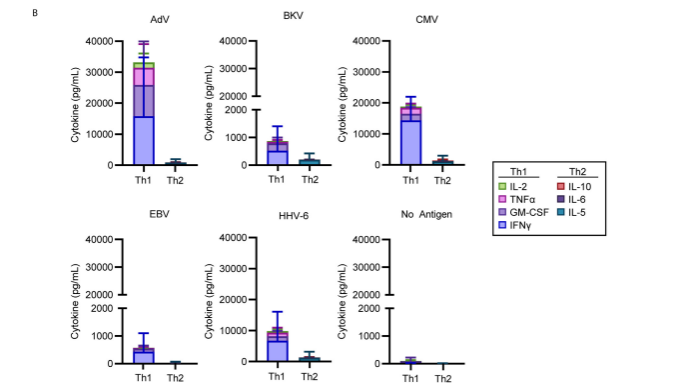
Figure 1. Against each of the five viruses, a Th1 (and not a Th2) cytokine response was activated when posoleucel cells were exposed to viral antigens.
The indirect evidence of cytolytic potential is shown in Figure 2, below.
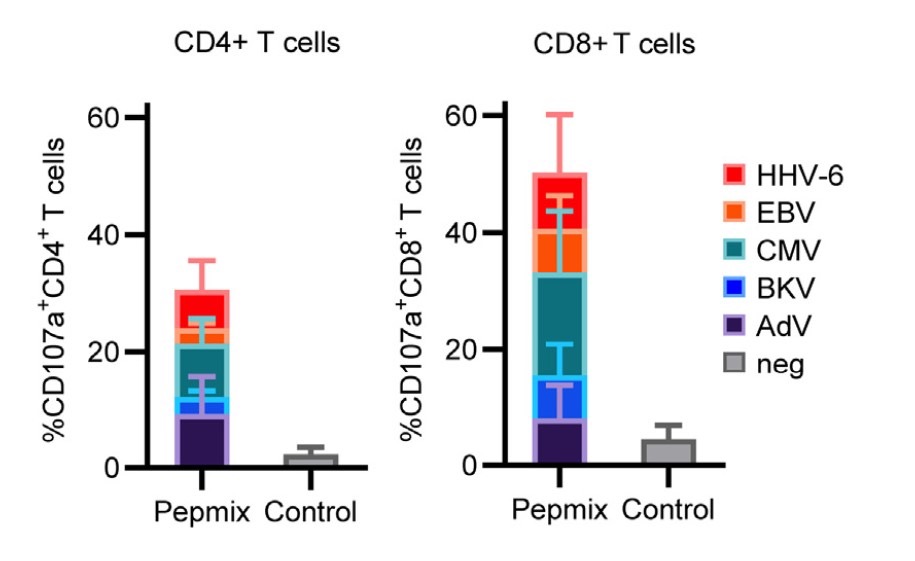
Figure 2. The mean frequency of CD107a expression on CD4+ and CD8+ T cells—a surrogate marker of degranulation and cytolytic potential) from five posoleucel cell lines, as determined by flow cytometry after stimulation with AdV, BKV, CMV, EBV, and HHV-6 either by viral antigens (Pepmix) or unstimulated (Control).
Many of these investigators then studied the use of posoleucel in patients undergoing allogeneic hematopoietic cell transplantation (Dadwal 2024). The team conducted an open-label, single-arm, phase 2 study of 26 patients at high risk for clinically significant opportunistic infections (not merely reactivated virus) who were undergoing allogeneic HCT. The therapy was infused within 15-49 days of transplantation. In vivo expansion of functional virus-specific T cells was documented by interferon-γ enzyme-linked immunosorbent spot assay. Using T-cell–receptor deep sequencing, the team also documented persistence of posoleucel-derived T-cell clones for up to 14 weeks after the last infusion.
Three patients (12%) developed a clinically significant infection, each with a single virus. Five patients (19%) had acute graft-versus-host disease grade 2 to 4. No patient experienced cytokine release syndrome. Six patients died from relapse or disease progression. Repeat posoleucel dosing was generally safe and well tolerated and associated with functional immune reconstitution. Adverse effects were relatively few, and are summarized in Table 1, below.
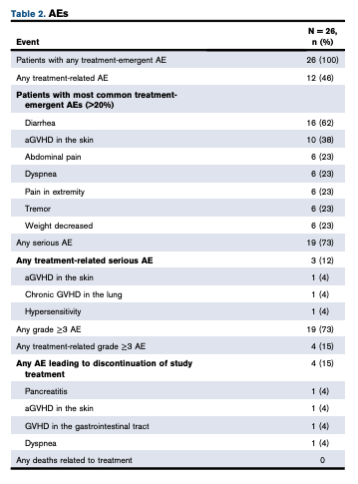
Table 1. List of adverse events
This study suggests that this form of antiviral cell therapy might provide clinical benefits—a reduction in clinically significant opportunistic infections—in patients undergoing allogeneic hematopoietic cell transplantation (HCT) at high risk for such infections. The study also suggests that the therapy has relatively few serious potential adverse effects.
However, proof of such benefit requires a randomized, controlled trial. Indeed, four such trials were initiated. Unfortunately, although the results have not yet been published, the trials were stopped after three independent review panels performing an interim review concluded that posoleucel would fail to meet their primary endpoints. In a press release, the company, Allovir, admitted that they also lacked sufficient capital to bring all of the trials to completion. After laying off 95% of their workforce, Allovir recently agreed to merge with Kalaris; all work on T-cell therapy was discontinued.
The failure was a disappointment to transplant physicians as well as to Ann Leen, PhD and her team at Baylor’s Center for Cell and Gene Therapy, who developed the therapy and served as Chief Scientific Officer of Allovir. Although this particular approach to “off-the-shelf”, virus-specific memory T cell therapy for preventing and treating viral reactivation in immunosuppressed or immunocompromised patients failed, the failure in no way justifies abandoning a theoretically promising approach. Novel approaches to therapy may take decades of modifications to achieve the desired efficacy and safety.
Read the full text: Vasileiou 2024; Dadwal 2024

