Germline integration (and thus inheritance) of HHV-6A/B into telomeres (iciHHV-6A/B) is found in approximately 1% of the population (Arbuckle et al. 2010; Arbuckle et al. 2013)). Using a novel genomic biomarker, Wood et al. (2021), of the Department of Genetics and Genome Biology at the University of Leicester have made several new insights into the biology of iciHHV-6B.
Using the hypervariability of DRr-pvT1 region of the HHV-6B genome as a genetic biomarker helped the investigators distinguish iciHHV-6B from acquired HHV-6B (acqHHV-6B), and helped monitor transmission of iciHHV-6B and acqHHV-6B between family members. As would be expected, over three generations, the region was found to be very similar among family members with iciHHV-6B. In contrast, the region was quite variable in acqHHV-6B from individuals without iciHHV-6B.
In saliva DNA analysis of 34 individuals from eight families without iciHHV-6B, the same DRr-pvT1 region sequence was found in children and parents in 18 of 20 cases, presumably because acqHHV-6B had been transmitted (at birth or later in childhood) from a parent, typically the mother.
Using the DRr-pvT1 marker, the team demonstrated transmission from an iciHHV-6B mother to her non-iciHHV-6B son. This implies that in the mother the chromosomally integrated virus had been excised, and produced full virions that were transmitted to the son.
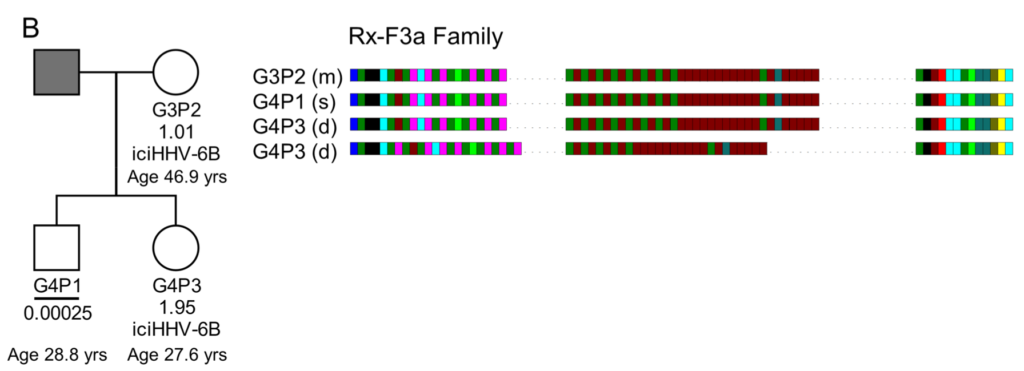
This family tree demonstrates reactivation of iciHHV-6B through the transmission from an iciHHV-6B mother (G3P2) to her non-iciHHV-6B son (G4P1). The DRr-pvT1 repeat patterns are shown and the son (G4P1) was found to have the exact profile as the mother (G3P2) (this repeat pattern was not seen outside this family among 102 DRr-pvT1 repeat patterns). The copy number of HHV-6B per cell in saliva DNA of G4P1 (0.00025) is also shown. This number is consistent with acqHHV-6B (not iciHHV-6B) infection. - Wood et al. 2021
Other interesting findings from this study, based on the use of the DRr-pvT1 marker, included:
- Showing that mutations occur in the DNA of the inherited virus in people with iciHHV-6;
- That these mutations can produce mosaicism (variability of the viral genome) in different tissues of people with iciHHV-6B, indicating tissue-specific mutational events;
- Finding circular extra-chromosomal forms of HHV-6B that have the potential to replicate;
- Demonstration that some copies of acqHHV-6B found in saliva had gained a telomere, indicating past integration and latency;
- Demonstration that the frequency of viral genome excision from telomeres in people with iciHHV-6B is surprisingly high;
- Demonstration of integration of acqHHV-6B into telomeres, using Single Telomere Length Analysis (STELA), although it remains unclear how commonly the acquired virus might achieve latency in this way;
- That when inherited virus is excised from the telomeres, thereby shortening the telomere, the telomere is then lengthened by the action of telomerase in those cells that can activate telomerase.
Together, these various findings indicate that iciHHV-6B can easily transition between the telomere-integrated form and the free virus form.
In addition to these biological findings, the authors note at least one possible clinical application of the the DRr-pvT1 marker: in the setting of organ transplants, it may help to distinguish reactivation of iciHHV-6B vs. acqHHV-6B by the recipient, a capability that transplant physicians have been enthusiastic about (Hill, 2019).
Read the full article: Wood et al. 2021