A combination of studies find that both reactivation and disease are infrequent after CAR-T therapy.
A recent publication in Nature (Lareau 2023) reported that one source of the HHV-6B that reactivates during CAR-T cell therapy are the infused CAR-T cells, themselves. Using single-cell sequencing, they demonstrated that a small group of cells (0.01% and 0.3% of the cultured CAR-T cells) become “super-expressors” of HHV-6B. After infusion into patients, the virus reactivates, proliferates, and spreads to other cells. As a result, extending the time of cell cultures leads to an exponential increase in the amount of virus.
While occasional case reports of HHV-6-related complications following CAR-T cell therapy (primarily encephalitis) have been reported, it has not been clear how often HHV-6-related complications occur in patients undergoing CAR-T therapy. One common complication of CAR-T cell therapy is immune effector cell-associated neurotoxicity syndrome (ICANS), a condition with symptoms that overlap with the neurologic dysfunction associated with HHV-6B reactivation.
A team from the Fred Hutchinson Cancer Center, led by Joshua Hill, conducted two studies to address this question. The first was a prospective study involving weekly HHV-6B testing for up to 12 weeks after CAR-T cell infusion. HHV-6B viremia was found in 8/89 (9%) subjects, 3 of whom had iciHHV6; thus, 6/89 were clearly reactivated virus. In the cases of reactivation, viral loads were low and none of the patients had clinical findings warranting antiviral therapy. Of interest, all six patients with HHV-6B reactivation had preceding cytokine release syndrome (CRS) and three had preceding or concomitant ICANS.
The clinical course in the patients with reactivated HHV-6B—including ICANS and CRS—treatments given and outcomes (relapse or death) in the 6 patients with reactivation of HHV-6B is shown in the Figure, below. Each row depicts an individual participant.
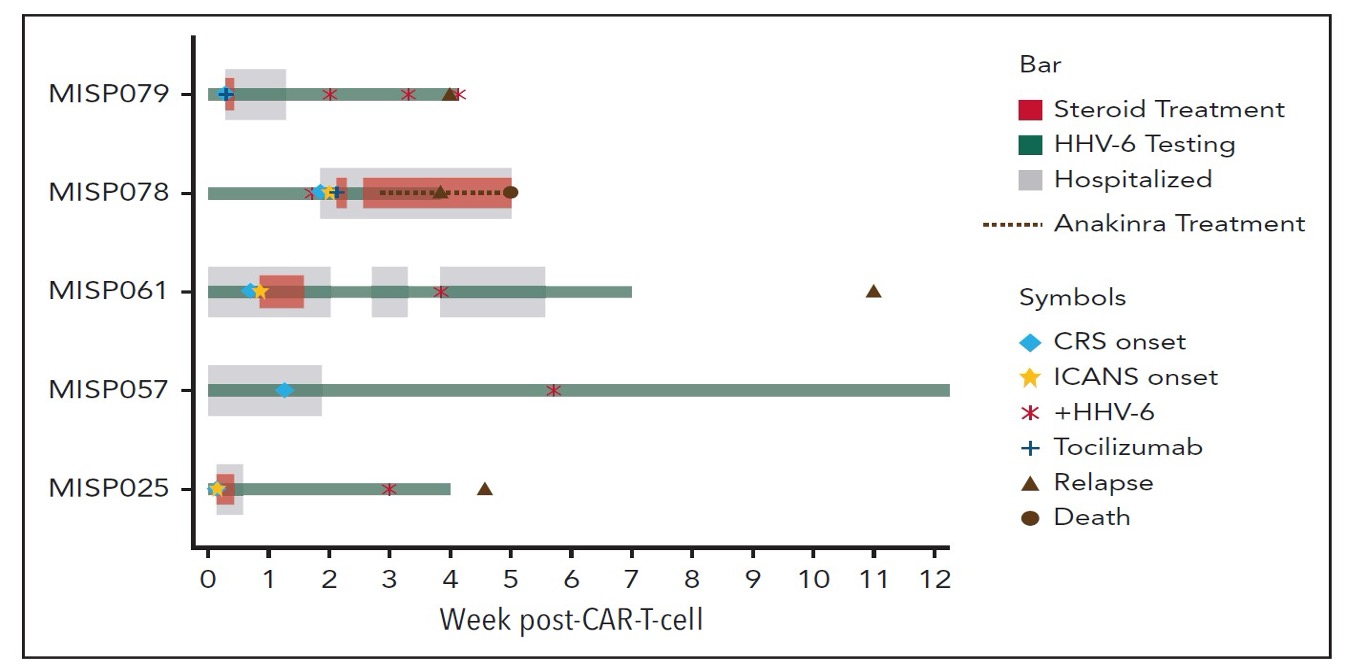
Figure. Clinical course of participants with HHV-6 reactivation after CAR-T therapy.
The second study involved retrospectively analyzing data from 626 patients who had undergone CAR-T cell therapy. During 12 weeks following the cell therapy infusion, symptoms leading to plasma testing for the virus occurred in 24 patients, with virus detected in just one of the 24. Among 34 patients in whom cerebrospinal fluid (CSF) was obtained because of clinical findings, one patient had possible HHV-6 encephalitis—a condition that improved without antiviral treatment.
Although the preparation of CAR-T cells for therapy can sometimes induce cells that are super-expressors of HHV-6B, these two studies indicate that this happens infrequently. Nevertheless, further prospective longitudinal studies are in order to better understand and to reduce the threat.
Specifically, important questions remain to be pursued:
- Are some cases of pneumonia, cytokine release syndrome, and bone marrow depression post-CAR-T cell therapy related to HHV-6B super-expressor CAR-T cells?
- Will identifying CAR-T cell products that contain super-expressor cells, and treating them with antivirals prior to infusion, reduce the frequency of adverse clinical events after the CAR-T therapy?
Read the full article: Kampouri 2024; Peggs 2024