When patients are tested for HHV-6 DNA—typically patients with hematologic malignancies or other diseases being treated with hematopoetic cell transplantation (HCT)—detection of a high viral load can lead to the assumption that the patient has a severe active/reactivated infection. Chromosomally-integrated HHV-6 (iciHHV-6) individuals always have elevated viral loads, with or without an active infection.
Heldman et al. (2021) of the Fred Hutchinson Cancer Center conducted an observational study of patients in the first 100 days following allogeneic HCT. Diagnostic testing and treatments were chosen by clinicians, and not by a study protocol. The study compared 87 patients with (iciHHV-6) vs. 174 comparison patients (matched by various factors) without iciHHV-6.
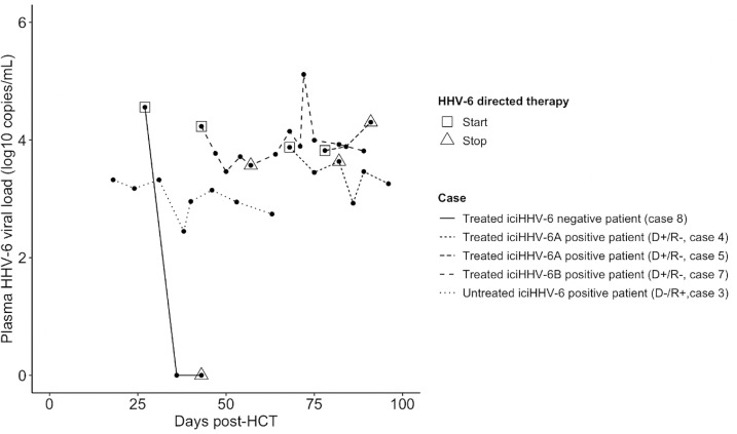
Plasma HHV-6 viral loads in response to HHV-6 directed therapy for 4 iciHHV-6A,pos patients and 1 iciHHV-6,neg patient.
The cumulative incidence of CNS symptoms was similar in the two groups. However, HHV-6 DNA tests were, as would be expected, much more likely to be positive in the iciHHV-6 group than in the comparison patients: in the plasma, 100% vs 13% were positive. Particularly in patients seen before there was widespread appreciation of the existence and implications of iciHHV-6, this led to more diagnostic testing (often judged unnecessary) and treatment in the iciHHV-6 group, with some adverse events. For example, iciHHV-6 was associated with a 12-fold increase in antiviral use (OR, 12.81; 95% CI, 1.52 - 108.2), and several patients experienced significant toxicities from HHV-6-directed treatments.
Previous studies have shown that that iciHHV6 transplant patients have increased rates of CMV viremia and acute graft vs host disease (Hill 2017). Unfortunately standard CSF and plasma HHV-6 PCR testing cannot distinguish between integrated genomes and actively replicating virus. Cellular samples, on the other hand can identify iciHHV6 patients easily. A whole blood qPCR result of a ciHHV6 individual typically exceeds log 5.5 log 10 copies/mL (Pellett 2012).
In this study, the presence of iciHHV-6 was determined using digital droplet polymerase chain reaction (ddPCR), a technology that currently is not widely available, but offered through the clinical lab at University of Washington. Most commercial laboratories in the US perform HHV-6 qPCR testing exclusively on plasma.
The authors note that none of the patients in this retrospective study received UCB, HLA donor-recipient mismatch or ex vivo T cell depletion transplants, all of which confer a higher risk of HHV-6 reactivation, delirium and limbic encephalitis.
They conclude that reflexive or targeted testing for iciHHV-6 may aid in post HCT management and reduce the cost and complications of antiviral treatment.
Read the full article: Heldman et al 2021

