A growing number of case reports reveal reactivation similar to that seen after hematopoietic stem cell therapy. Could CAR-T cells be a source of lytic HHV-6?
HHV-6 encephalitis is a well-established complication of the severe immunosuppression of hematopoietic stem cell therapy (HSCT). Chimeric antigen receptor T cell therapy (CAR-T) also generates severe immunosuppression, and previously has been reported to lead to HHV-6 associated myelitis (spinal cord inflammation) (Handley 2021). Two papers in the past year describe cases of HHV-6 encephalitis post CAR-T cell therapy and at least ten cases have been reported.
For example, a case report published in the New England Journal of Medicine (Spanjaart 2022) described one such case of one such CAR-T cell patient who developed confusion, developed psychosis after glucocorticoid treatment, and was ultimately diagnosed with treated for HHV-6 encephalitis.
An earlier report (Rebechi 2021) linked CAR-T cell therapy to HHV-6B-associated encephalitis in two cases. Evidence of encephalitis emerged about one month after the CAR-T cell therapy, and was documented by finding HHV-6 DNA in spinal fluid and magnetic resonance imaging (MRI) changes similar to those seen in post-HSCT HHV-6 encephalitis.
The authors point out that HHV-6 encephalitis in CAR-T cell therapy can be hard to distinguish from a clinical entity called immune effector cell-associated neurotoxicity syndrome (ICANS), an entity previously reported in people undergoing CAR-T cell therapy. Compared to HHV-6 encephalitis following HSCT, ICANS in CAR-T cell therapy tends to occur sooner (3-10 days following therapy), is not associated with HHV-6 DNA in spinal fluid, more often occurs with cytokine release syndrome and fever, and has a more rapid progression. The distinction is summarized in the Table, below.
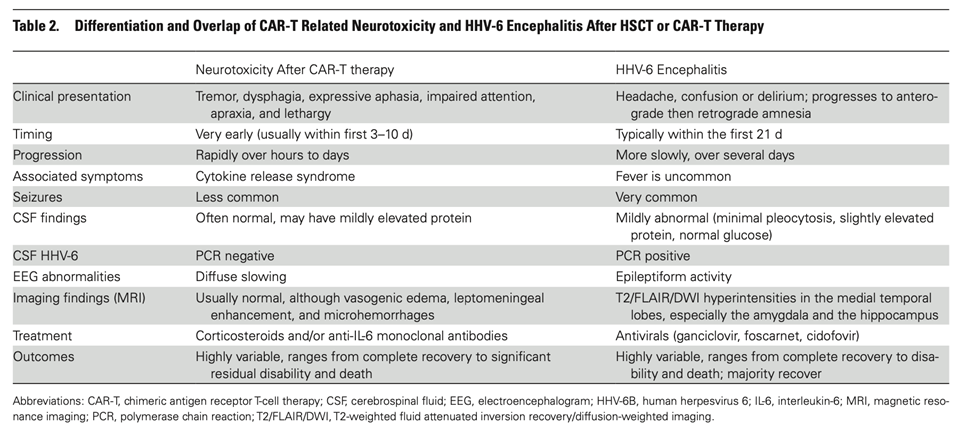
Making the distinction may be important because ICANS probably benefits from corticosteroid or anti-IL-6 monoclonal antibody treatments, whereas HHV-6 encephalitis probably benefits from antiviral therapy or adoptive cell therapy (the absence of large randomized trials makes the value of these treatments uncertain). The authors recommend a low bar for HHV-6 spinal fluid testing. Currently, lumbar punctures are not routine as most patients are assumed to have ICANS.
Could some of these CAR-T cells contain actively replicating HHV-6? A recent bioRxiv preprint from Stanford claims that the answer may be yes. Allogene, a company developing off the shelf CAR-T cells, supported a team to research this question. They found a rare population of HHV-6 “super-expressors” with high viral transcriptional activity, and conclude that cell therapy products may be the source of lytic HHV-6 reported in clinical trials.
Read the full articles:
Laureau 2022 (preprint)

