Japanese study replicates the paradoxical results of a prior US study, but fails to explain the paradox.
Since 2017, transplant centers have started using a CMV-specific antiviral, letermovir, to prevent CMV reactivation instead of more toxic broad-spectrum antivirals (e.g., valganciclovir, cidofovir, foscarnet) following hematopoietic cell transplantation (HCT). Since broad-spectrum antivirals have been shown to reduce rates of HHV-6B reactivation post-HCT, and since letermovir was not active against HHV-6B, there was concern that switching would lead to increased rates of HHV-6B reactivation and encephalitis.
Surprisingly, a 2023 retrospective observational study from the University of Washington found no evidence that letermovir prophylaxis led to increased reactivation of HHV-6B or related complications (Kampouri 2023). One of that study’s limitations was that it included relatively few people with cord blood transplants (CBT), a type of transplant with a higher risk of HHV-6B reactivation.
A new multi-center observational study from Japan that includes more CBT patients, however, comes to the same surprising conclusion. The study included 7985 patients between ages 18-75 who were receiving their first allogeneic transplantation. Patients fell into three groups: (1) broad-spectrum antiviral drugs including prophylactic foscarnet, ganciclovir or valganciclovir; (2) oral or intravenous letermovir; and (3) other antiviral drugs.
The primary endpoint was the incidence of HHV-6 encephalitis by day 100 post-transplant. HHV-6 encephalitis occurred in 3.6% of the patients. It occurred in 11.5% for the broad-spectrum antiviral group; 2.8% for the letermovir group and 3.8% for the other antiviral group, a highly significant difference (p < 0.001).
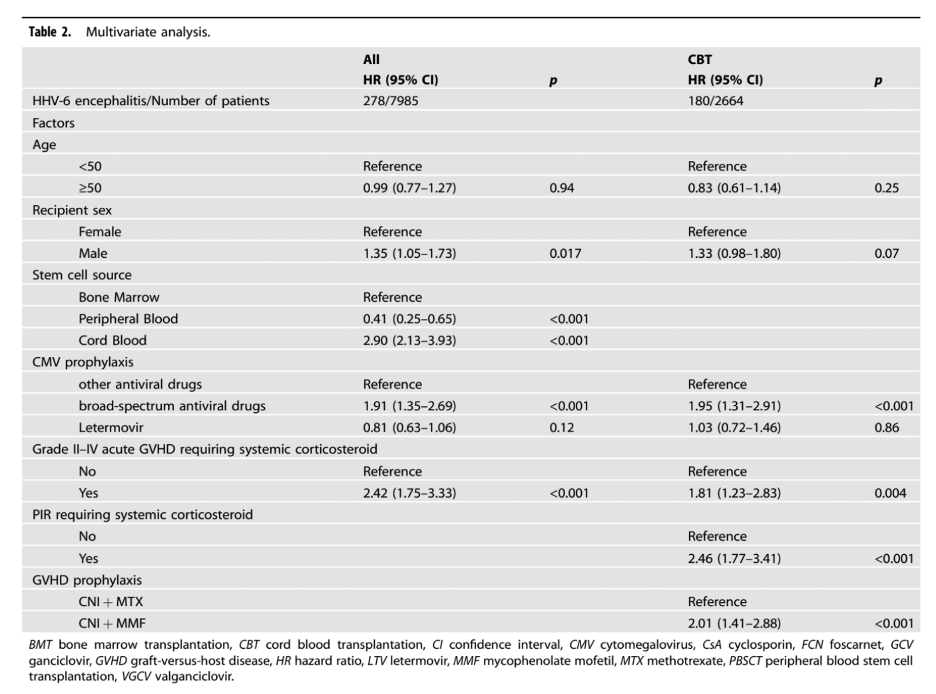
HHV-6 encephalitis was more likely to occur in cord blood transplants (CBT) than in bone marrow transplants (BMT) or peripheral blood stem cell transplants (PBSCT)—as has been found in prior studies. For that reason, a separate analysis was conducted just of the CBT patients, and the same differences persisted: HHV-6 encephalitis occurred in 14.1%, 5.9%, and 7.4% of the three groups, respectively (p < 0.001).
In the multivariate analysis, several factors increased the risk of HHV-6 encephalitis: CBT (hazard ratio [HR]: 2.90), broad-spectrum antiviral prophylaxis (HR: 1.91), and grade II–IV acute graft-versus-host disease requiring systemic corticosteroids (HR: 2.42). Each of these independent risk factors for encephalitis were highly significant (all p < 0.001).
The results for the full population, and for just the CBT patients, are summarized in Table 2, below.
Thus two large and excellent studies, involving very different populations (ethnically and socially), have come to a surprising conclusion: despite theoretical reasons for believing the use of letermovir rather than broad-spectrum antivirals for HCMV prophylaxis would increase the risk of HHV-6B encephalitis, that does not appear to be happening.
There are several possible explanations of this paradoxical result:
- Perhaps CMV reactivation itself triggers reactivation of HHV-6: the efficacy of letermovir in preventing CMV reactivation thus may also have suppressed HHV-6 reactivation.
- Perhaps because letermovir is less myelotoxic than broad-spectrum antivirals, and thereby improves immune reconstitution, the resulting more vigorous immune response following letermovir therapy is what inhibits reactivation of HHV-6B.
- Perhaps, despite using statistical techniques in both studies to eliminate unmeasured underlying differences in the patients pre- vs. post-letermovir, such differences nevertheless were present.
- Perhaps because letermovir is better able to penetrate the blood-brain-barrier than the broad-spectrum antivirals, it is more effective.
The authors note several weaknesses of the study, including the fact that it was a retrospective multi-center registry study, with the centers following different protocols. Importantly, many key data points were unknown, such as the dosage of the prophylactive drugs used, severity of cytopenia, viral load and concomitant medications. Finally, they note that selection bias may have occurred as patients with other unknown risk factors may have been more likely to receive broad spectrum antivirals.
As did the prior study (Kampouri 2023), this new study underlines the importance of funding a prospective, controlled trial to better assess the impact of letermovir prophylaxis on immune reconstitution, the reactivation of both CMV and HHV-6, and on resulting encephalitis, pneumonitis and other sequelae.
Read the full article: Terao 2024

