Greatly increased risk of death, acute GVHD, transplant-associated microangiopathy found.
HHV-6 encephalitis is an unusual but serious complication of allogeneic hematopoietic stem cell transplantation (HSCT). Several risk factors for the development of this disease have been proposed, and several adverse outcomes associated with the disease have been suggested, by prior studies. However, the small size of the studies has made firm conclusions difficult, leading Chinese investigators to perform a larger study of adult patients.
Investigators from multiple institutions in Zhejiang Province reviewed 1350 consecutive patients following allogeneic peripheral blood stem cell transplantation (PB-HSCT) between 2018 and 2022, and looked for cases of HHV-6 encephalitis, the definition of which required that a patient:
- Had experienced neurologic symptoms consistent with encephalitis (such as disorientation as to time and place, loss of consciousness, abnormal behaviors, memory loss, convulsions, or dysesthesia not attributable to peripheral neuropathy);
- Had HHV-6 DNA detected in the cerebrospinal fluid; and
- Had no other cause of neurological dysfunction.
The investigators identified 20 (1.48%) patients with HHV-6 encephalitis, which developed at a median onset time of 25.5 days after HSCT. Then they matched four control subjects to each case who were of the same gender and whose transplantation occurred a week before or after the transplantation date of the case. The investigators then performed a nested case-control cohort study to evaluate risk factors and long-term outcomes, using multivariate analysis to control for possible confounding factors.
Two independent risk factors for developing HHV-6 encephalitis were identified in the multivariate analysis: patient age < 30 (odds ratio [OR], 3.24, P=0.016) and a low NK cell count (< 115 cells/μl) at 21 days (OR, 6.07, P=0.018).
The investigators then asked whether outcomes were different in the unusual patients who developed HHV-6 encephalitis. They found that the following adverse outcomes were significantly more likely:
- Grade II-IV graft-versus-host disease (aGVHD) (hazard ratio [HR], 5.52, P < 0.001);
- Transplant-associated microangiopathy (TAM) (HR,9.86, P < 0.001);
- Higher non-relapse mortality (NRM) (HR, 5.28, P=0.004);
- Lower overall survival (OS) (HR, 4.34, P=0.001); and
- Lower progression-free survival (PFS) (HR, 3.94, P=0.001).
These outcomes in the patients who developed HHV-6 encephalitis and the matched control subjects are shown graphically in Figure 1.
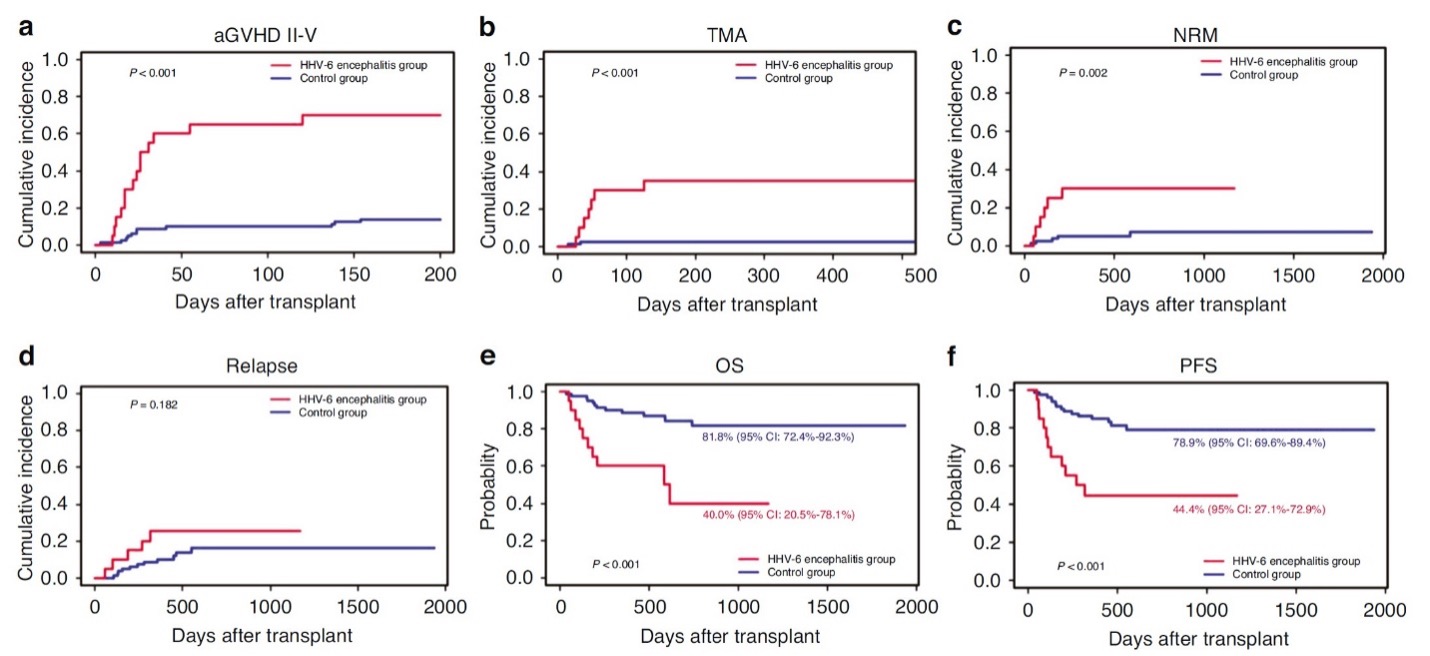
Figure 1. Outcomes in allogeneic peripheral blood stem cell transplantation patients with vs. without HHV-6 encephalitis. aGVHD = grade II-IV graft-versus-host disease; TAM = transplant-associated microangiopathy; NRM = non-relapse mortality; OS = overall survival; PFS = progression-free survival.
Fever (70%), confusion (55%), sensory disorders (45%), syndrome of inappropriate antidiuretic hormone (SIADH) (50%) short term memory loss (45%) and seizures (25%) were the key clinical manifestations.
The high incidence of TMA (35%) and SIADH (50%) were surprising. A large study of 23,665 cases in the US found the incidence of thrombotic microangiopathy to be much lower at 3% (Epperia 2020). SIADH has recently been associated with HHV-6 infection, and the incidence in transplantation was reported to be 11% in one Japanese study (Kobayashi 2004).
This study confirms several prior studies that have found HHV-6 encephalitis is more likely in younger patients undergoing allogeneic peripheral blood stem cell transplantation. It also finds that patients with slower reconstitution of NK cells (lower NK cell counts) also are more likely to develop HHV-6 encephalitis.
The study also supports and extends prior studies that have concluded that patients with HHV-6 encephalitis are more likely to have several important adverse outcomes: more frequent grade II-IV graft-versus-host disease; more frequent transplant-associated microangiopathy; greater non-relapse mortality; poorer overall survival and poorer progression-free survival.
The 20 HHV-6 encephalitis patients were given antiviral therapy (ganciclovir or foscarnet) and 13 (65%) had a resolution of symptoms in response to treatment (controlled fever, reduced itching, improved mental symptoms, etc.) A randomized, controlled trial of therapy in people with HHV-6 encephalitis following HSCT, particularly PB-HSCT or cord blood HSCT, would help address the question of causality—as well as establishing whether treatment protects against these important adverse outcomes.
Read the full article: Yu 2024

