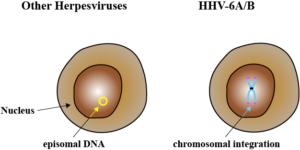
Most herpesviruses maintain latency by forming circular episomes in the nucleus of the cell. Investigators in Germany have provided further evidence that HHV-6A relies on their telomeres, not circular episomes, to maintain a persistent latent infection by integrating into the host chromosome. HHV-6A genomes missing their telomeric repeats could replicate but they could not enter latency.
 Investigators led by Benedikt Kaufer at the Freie Universität in Berlin created a mutant HHV-6A virus that lacked the telomeric repeats. They found that the virus could replicate well without the repeats, but the defective virus was not able to integrate and was quickly lost. The authors also revealed the mechanism by which HHV-6 integrates, which was previously unknown.
Investigators led by Benedikt Kaufer at the Freie Universität in Berlin created a mutant HHV-6A virus that lacked the telomeric repeats. They found that the virus could replicate well without the repeats, but the defective virus was not able to integrate and was quickly lost. The authors also revealed the mechanism by which HHV-6 integrates, which was previously unknown.
In addition to HHV-6A, 16 other herpesviruses have telomeric repeats at the tips of their chromosomes that could permit chromosomal integration. This has only been confirmed for three: Marek’s, HHV-6A, and HHV-6B. There was also a recent report of rare HHV-7 chromosomal integration.
Roseoloviruses are the only human herpesvirsuses with telomeric repeats capable of chromosomal integration. The best studied herpesvirus with this capability is Marek’s disease virus, a highly oncogenic alpha herpesvirus that infects chickens. Integration of Marek’s virus is a prerequisite for lymphoma formation in the chicken.
The investigators provided conclusive evidence to support previous reports that whether it is inherited (iciHHV-6) or community acquired, HHV-6 uses its telomeres to facilitate reactivation from latency. The authors note that integration not only provides a stable environment for HHV-6A persistence, it permits a more efficient mobilization out of a latent state during reactivation (Kaufer 2011).

Benedikt Kaufer, Associate Professor Freie University, Berlin, Germany
HHV-6 is the first herpesvirus to reactivate after immunosuppression and also the most prevalent herpesvirus infection in the cord and stem cell transplantation settings. In one Japanese study that used a multiplex assay to study 13 common viruses in stem cell transplant patients weekly, 20% had HHV-6B reactivations by 14 days, and over 40% had reactivated by 21 days, whereas significant CMV and EBV infections did not occur until after 28 days (Inazawa 2015). Could this be due in part to their uniquely efficient form of latency?
The authors point out that much work remains to be done to fully understand the mechanisms of integration and maintenance of cells latently infected with integrated herpesvirus.
Read the full paper: Wallaschek 2017

