A new in vitro study from the lab of Bhupesh Prusty, PhD at the University of Würzburg shows that HHV-6A direct repeats can survive alone in an integrated state without the rest of the viral genome. The study also identified non-telomeric integration of HHV-6A in both in vitro cultured cells as well as one iciHHV-6A patient with cardiac disease, suggesting a possible novel mechanism for HHV-6A mediated human diseases.
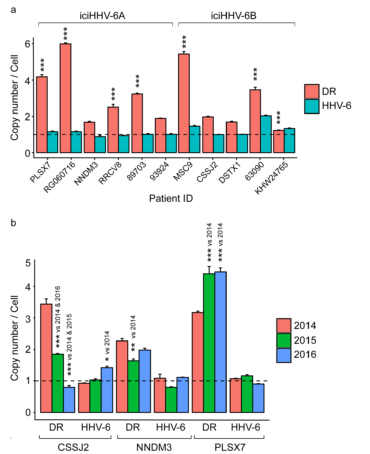
DR vs. HHV-6 genome copies for iciHHV6 individuals. Source: Nature Scientific Reports
In some of the cells, the investigators found that the integrated viral genome was excised from the chromosome, leaving behind only the viral direct repeats (DR). In U-251 glioblastoma cell clones, 32% retained multiple DRs in the absence of the rest of the HHV-6 genome. In fresh blood samples from iciHHV-6 individuals, they found that 3 of 11 patients had 5-6 DR copies for every 1 integration.
Viral DR is a unique region of the viral genome that encodes proteins like DR6, which interacts with p53. DR6 has been described as an “oncogene” because of its tumorigenic potential. Several viral miRNAs are also expressed from HHV-6 DR, thus supporting the role of viral DR in potential disease progression. The authors suggest that it is plausible that DR alone could contribute to clinical conditions even in the absence of the rest of the viral genome. Over two dozen individuals with inherited DR were previously reported in a screen of over 23,000 participants in Scotland (Bell, 9th International Conference on HHV-6 & 7
The study, which analyzed the status of viral integration after infection, found that cells can retain HHV-6A integration to different extents depending upon cell type. For example, 34% of HeLa and 80% of ovarian cancer cells lost the genome integration within ten passages.
The authors noticed unique non-telomeric integration of iciHHV-6A in several clones. That led them to search for non-telomeric integration in the iciHHV-6 patients, and they identified one individual with non-telomeric integration at chromosome 5q13.3. Further analysis suggested that the integration occurred in a region that plays a key role during angiogenesis. The authors note that the rare non-telomeric integration of ciHHV-6A could be a cause or consequence of chromosomal rearrangement.
IciHHV-6 was recently found to be associated with an increased risk of angina (Gravel 2015).
Read the full paper: Gulve 2017.