Ribavarin, ritonavir, abacavir and lamivudine were the primary triggers.
The drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a severe illness that has been linked repeatedly to benzodiazepines, antibiotics (particularly beta-lactams) and anti-convulsants. Reactivation of several herpesviruses, including HHV-6 and HHV-7, is often present in the plasma of these patients, typically 10-14 days after rash onset. Although there is little evidence that viral infections can trigger DRESS, the question of whether viral infection exacerbates the syndrome and contributes to organ damage is debated. Regardless, antiviral drugs sometimes are used to treat DRESS.
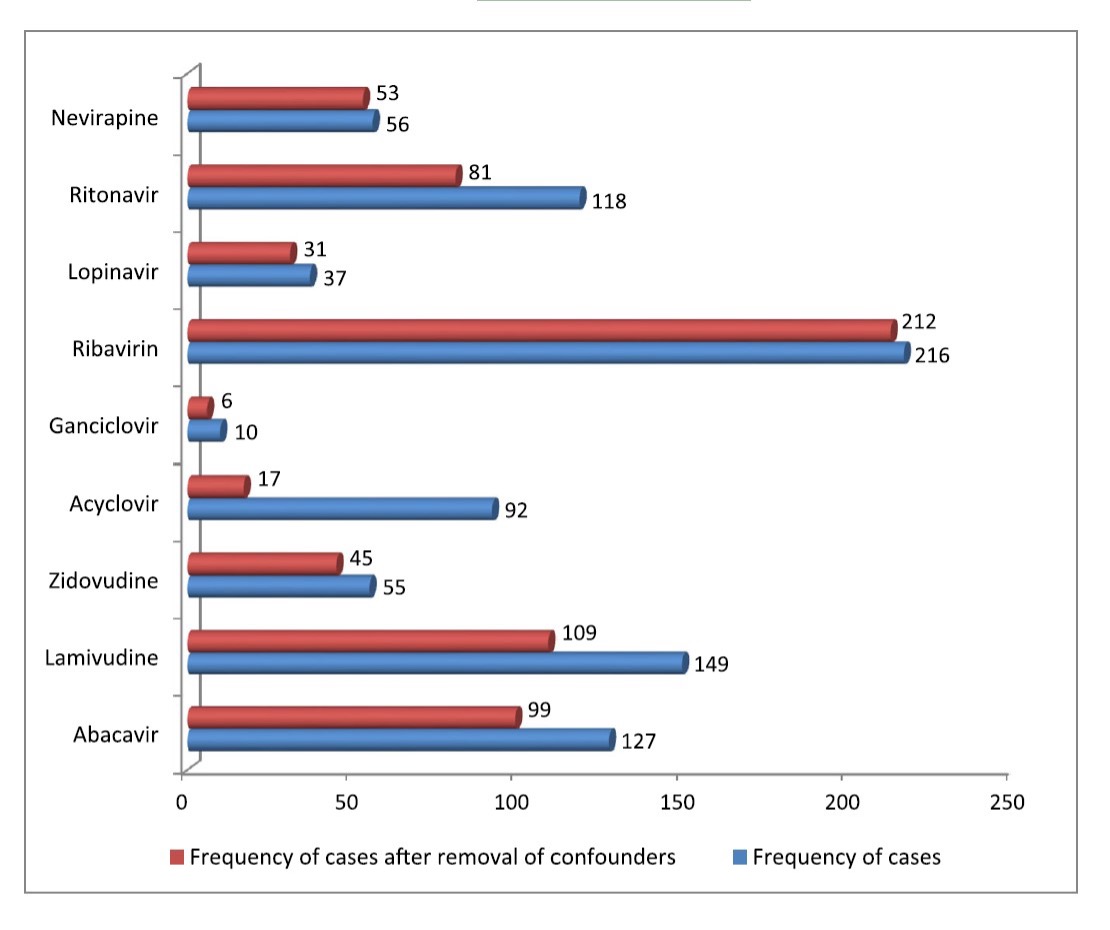
A group from New Delhi, India, examined the U.S. Food and Drug Administration (FDA) adverse event reporting system (FAERS) database, using established data mining tools, to see if any antiviral drugs might be associated with DRESS. Of 12,369 cases of DRESS in the database, 1218 cases were associated with antiviral drugs. In some of these cases, patients also were taking other drugs—including some previously linked to DRESS. So statistical techniques were used to determine if the association of several antivirals with DRESS was valid.
After this statistical adjustment, the antiviral drugs abacavir, ganciclovir, lamivudine, lopinavir, nevirapine, ribavirin, ritonavir and zidovudine were linked to DRESS. Although initially linked to DRESS, acyclovir no longer was incriminated after statistical adjustment. As shown in Figure 1, ribavirin was associated with the most cases.
Among the antivirals potentially linked to DRESS, only ganciclovir has in-vitro efficacy against HHV-6 commonly used to treat reactivated herpesvirus infections. Hence, when herpesvirus reactivation occurs with DRESS, and antiviral therapy is being considered, other drugs besides ganciclovir might be considered first.
Since HHV-6 reactivation doesn’t occur until 10 -27 days after presentation (Tohyama 2007), HHV-6 is unlikely to be a triggering event in DRESS. However, DRESS/DIHS patients with subsequent HHV-6 reactivation show an increased rate of severe disease and mortality (Tohyama 2007). The value of antiviral therapy for HHV-6 reactivation in DRESS/DIHS has not been studied in a large randomized trial.
This study underlines the importance of determining whether antiviral therapy speeds resolution of the syndrome. Some centers have encouraged further study on the role of HHV-6 antivirals for refractory DRESS with HHV-6 reactivation (Descamps 2013).
Read the full article: Sharma 2023

