Marmosets infected with HHV-6A/B intranasally were initially asymptomatic but later developed significantly accelerated disease and died in a shorter period of time compared to control animals in an MS-like model called experimental autoimmune encephalomyelitis (EAE). HHV-6 proteins were found at high levels in the brain lesions.
The authors conclude that the results support the role of viral infection in triggering “inflammatory-mediated neurologic conditions” like MS. In addition to “setting the stage” for autoreactivity, the heightened viral activity during the course appeared to promote increased lymphocytic infiltration into the brain, contributing to lesion development. Taken together, the data suggests that antiviral treatment could benefit patients with MS with persistent HHV-6A/B infection, and trials using antivirals in early stages of MS are justified.
There was intense lymphocytic infiltration in HHV-6+ lesions, and HHV-6 colocalized with CD3+ T cells. Compared to uninfected marmosets, a decrease in naïve CD8 T cells and increase in effector/memory CD8 cells in the blood (PBMCs) was characteristic of HHV-6A/B+ marmosets at the end of the EAE course, and this event was significantly associated with EAE duration in the infected animals.
Appropriate animal models for HHV-6A and HHV-6B infections have been hard to come by, but in 2013, Dr. Steven Jacobson, of the National Institute of Neurological Disorders and Stroke, and his colleagues determined that marmosets are suitable models for both species of HHV-6, and they also possess greater genetic, immunological, and CNS anatomical proximity to humans than do other animals, such as rodents. In early experiments, the group found that marmosets inoculated intravenously, but not intranasally, with HHV-6A developed neurological symptoms (Leibovitch 2013).
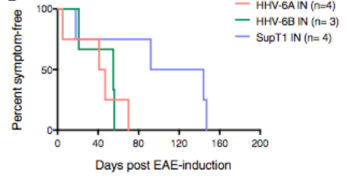
HHV-6A/B intranasal infection resulted in significantly reduced time to symptom onset.
In the present study, marmosets were used to model multiple sclerosis development. The animals were immunized with CNS peptides or proteins, which leads to development of experimental autoimmune encephalomyelitis (EAE), the animal model of MS. Once a month for 4 months prior to induction of EAE, the marmosets were inoculated intranasally with HHV-6A (n=4), HHV-6B (n=4), or uninfected SupT1 control materials, as this is thought to be a route of infection in humans. During these months, no animal developed clinical symptoms from the infections. Antibody responses (IgG in plasma) were observed against HHV-6B in 3/4 cases and against HHV-6A in 1/4. All marmosets inoculated with HHV-6B shed the virus in their saliva, as detected using ddPCR, while none of those with HHV-6A did, which is similar to what is seen in humans. One subject with HHV-6B was euthanized before EAE induction due to a non-HHV-6-related wasting disease, and when its brain was studied using immunohistochemistry, low level HHV-6 reactivity was observed in 2/9 brain regions, including the frontal cortex/olfactory bulb and hippocampus.
Two months after the last HHV-6 intranasal inoculation, EAE was induced, and marmosets that were infected with either virus developed EAE symptoms significantly faster than controls (p=0.04). The infected marmosets also survived for a significantly shorter period than controls (p=0.03). In addition, the infected subjects trended toward earlier brain lesion onset, as determined using MRI, had more acute inflammatory lesions, and exhibited a significantly shorter mean survival time after onset of lesions appearing on proton density-weighted and T1-weighted scans. Finally, the proportion of total lesions by volume that were T1 lesions was significantly greater among HHV-6-B+ marmosets than in HHV-6A+ or control marmosets. The greater proportion of T1 lesions, lesions that likely represented inflammation and tissue destruction, in HHV-6B+ brains may illustrate the tendency for HHV-6B to induce more acute inflammatory events in the CNS, such as encephalitis and seizures, compared to HHV-6A.
Unlike what was seen in the case of the HHV-6B+ marmoset that was euthanized and examined prior to EAE induction, upon immunohistochemical analysis of CNS tissues from a representative HHV-6B/EAE marmoset, HHV-6B late antigen p101K was detected in inflammatory, subcortical white matter lesions as well as in the leptomeninges around the hippocampal fissure and the choroid plexus. Viral antigen was up-regulated in EAE brain compared to the brain of the aforementioned animal without EAE.
Because MS lesions occur near blood vessels and result from inflammatory infiltrates flooding these areas, the permeability of the blood brain barrier is an important factor in regulating the infiltration and lesion development. Peripheral immune activation can affect permeability, and the preexisting HHV-6 infections in the marmosets may have induced this kind of activation and triggered expansion of proinflammatory memory/effector CD8+ cells after EAE induction. The authors propose that a heightened immune state spurred by HHV-6 infection can set the stage for expansion of autoreactive cells and thereby result in an enhanced response to a subsequent autoimmune challenge, thus reducing the threshold for MS development.
HHV-6 has been considered as a possible contributor to MS for many years, but a designation for HHV-6A/B as a causative agent has been elusive (Pormohammad 2018). In recent studies, HHV-6A has been found to impede myelin repair in vitro and in vivo, and both the U94 (Campbell 2017) and U24 (Sang 2017) gene products have been considered for roles in dysregulation of myelination, an important factor in MS severity. The data presented in the present study is the strongest evidence in favor of a causative/cofactor role for HHV-6A and HHV-6B in MS to date.
Find the full paper here: Leibovitch 2018.

