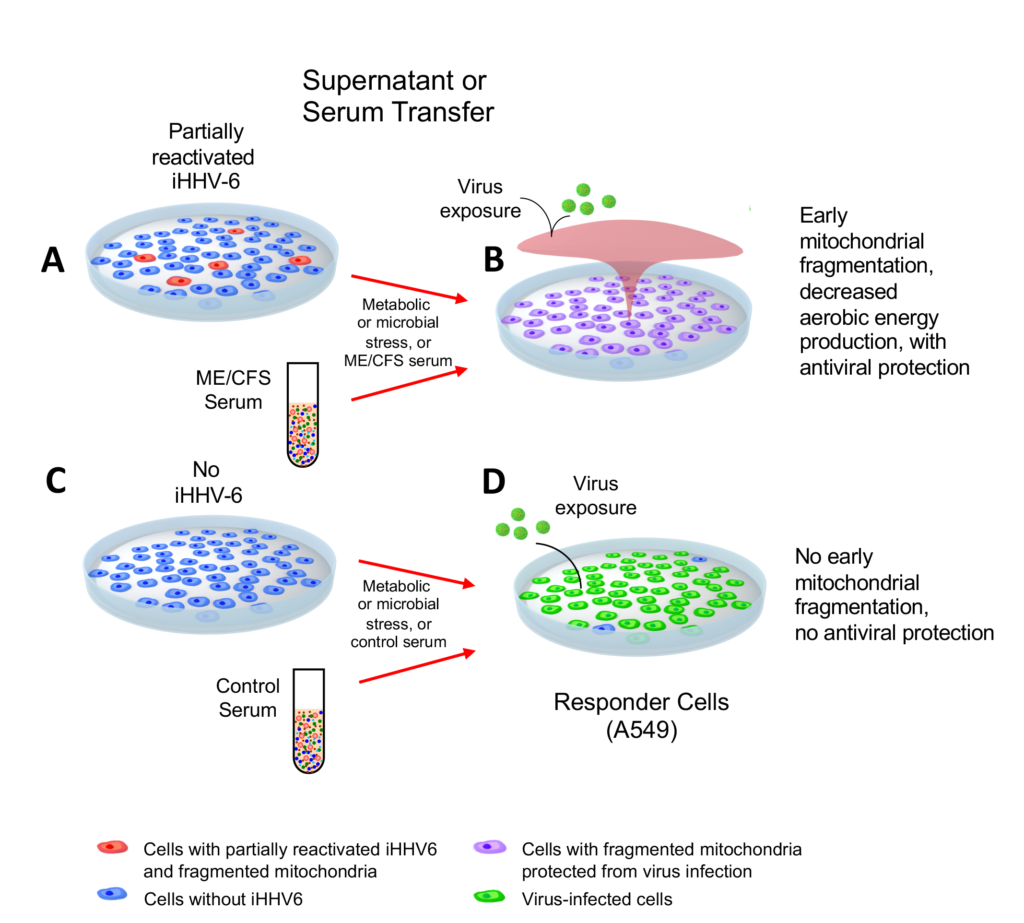
A team of virologists and metabolomics specialists collaborated to demonstrate how chromosomally integrated HHV-6 can be stimulated to secrete factors that simultaneously cause mitochondrial fragmentation, a reduction in intracellular ATP reserve and an expanded antiviral defense by their neighboring cells.
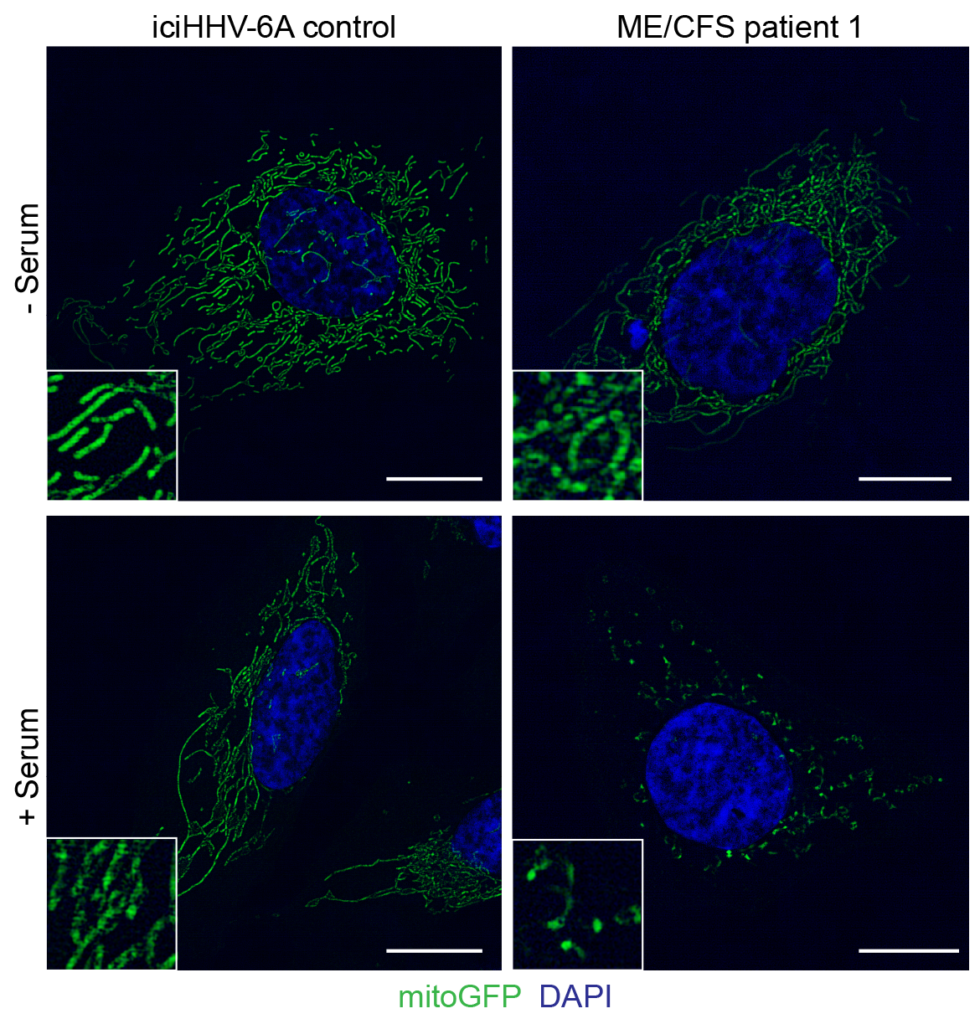
HHV-6A reactivation induces major changes in host cell mitochondrial architecture. Source: ImmunoHorizons, April 2020
The researchers from Julius-Maximilians University, in Würzburg, Germany and University of California, San Diego used trichostatin-A to activate chromosomally integrated HHV-6 cells, which were then challenged by infection with influenza-A or HSV-1. Adoptive transfer of the supernatant after HHV-6A reactivation led to an “antiviral state” that protected cells from subsequent infections. The authors refer to this phenomenon as the “cell danger response” (CDR).
The authors hypothesize that energy resources are reallocated in ATP production from the mitochondria to support defense against infection. Also, they found that actively replicating HHV-6 infection may not be necessary to cause this activity.
The components necessary for this transference of CDR are still unknown, but based on their tests, neither interferon nor tumor necrosis factor α appear to be involved. The authors also note that the CDR may not be specifically activated by HHV-6, but many other viral infections.
To connect these experiments to ME/CFS, the investigators tested to see if serum from ME/CFS patients could induce the same activity in naïve cells. They found that adoptive transfer of serum from 10 ME/CFS patients resulted in the same heightened antiviral state as was seen in cells exposed to the reactivated ciHHV-6A cell culture media.
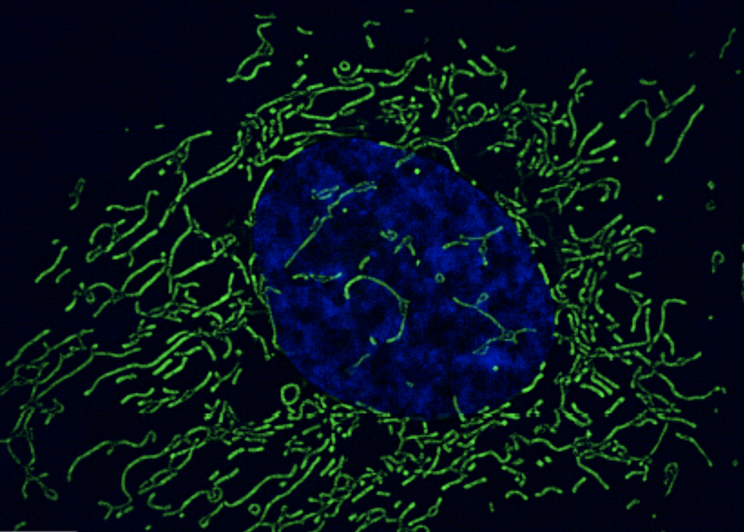
 Severe HHV-6B reactivation can trigger mitochondrial disease such as Alper’s- Huttenlocher syndrome syndrome in children (Al Zubeidi 2014). An earlier study (Yeo 2008) found that the U95 intermediate early gene of HHV-6B interacts with GRIM-19 (a gene that is an essential component of the oxidative phosphorylation system) to damage the mitochondria. Furthermore, silencing the HHV-6B U95 gene, eliminated the interaction with GRIM-19 and loss of mitochondrial function.
Severe HHV-6B reactivation can trigger mitochondrial disease such as Alper’s- Huttenlocher syndrome syndrome in children (Al Zubeidi 2014). An earlier study (Yeo 2008) found that the U95 intermediate early gene of HHV-6B interacts with GRIM-19 (a gene that is an essential component of the oxidative phosphorylation system) to damage the mitochondria. Furthermore, silencing the HHV-6B U95 gene, eliminated the interaction with GRIM-19 and loss of mitochondrial function.
UCSD issued a press release on the paper, and a popular ME/CFS newsletter also covered the paper.
Read the full text: Schreiner 2020