Karolinska Institute researchers, in collaboration with a group from Heidelberg, developed a novel serological assay to determine if individuals with antibodies to HHV-6A early proteins are more likely to develop multiple sclerosis. HHV-6A antibodies were highest in the presence of elevated EBV antibodies, suggesting that the two viruses could jointly contribute to the development of multiple sclerosis.

Anna Fogdell-Hahn, PhD, Associate Professor, Department of Clinical Neuroscience, Karolinska Institute
Previously, it wasn’t possible to differentiate between the ubiquitous HHV-6B acquired in early childhood and the less common HHV-6A that appears later in life. The Scandinavian group collaborated with the German Cancer Center to develop a novel bead-based multiplex serology assay that measured IgG antibodies against the most divergent early and late HHV-6A and B viral proteins.
Of interest, only HHV-6A immediate early-1 (IE1A) antibodies, which are associated with early or low-level activation, were markedly different, whereas the “late” antibodies associated with viral replication were not. Individuals with HHV-6A IE1A antibodies had twice the risk of developing multiple sclerosis (MS) than those without the antibodies.
The study, published in Frontiers in Immunology, covered 8,700 MS patients and 7,200 healthy controls. The earlier in life HHV-6A antibodies appeared, the higher the risk of developing MS in the future. Curiously, individuals with HHV-6B IE1 antibodies had a lower risk of developing MS than controls.
HHV-6 researchers have long sought a serological assay that could differentiate between HHV-6A and HHV-6B because the two have different disease associations. HHV-6B causes roseola, febrile seizures, febrile status epilepticus, and activates in transplant patients, while HHV-6A has been associated with neurological diseases such as MS and Alzheimer’s disease. HHV-6A has also been found in the thyroid tissues of patients with Hashimoto’s thyroiditis and the uterus of women with unexplained infertility (Marci 2016).
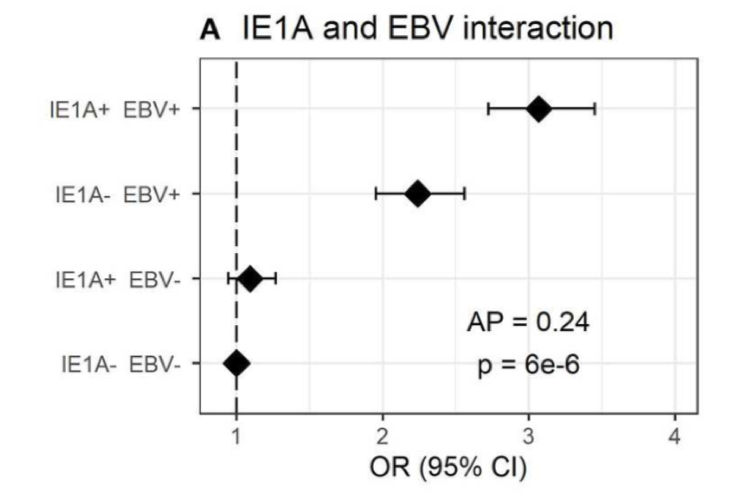
IE1A and EBV interacted with the highest odds ratio were for individuals with elevated antibodies to both HHV-6A IE-1 and EBV. Source: Frontiers in Immunology, November 2019
The authors point out that HHV-6A, but not HHV-6B, can establish a latent infection in oligodendrocytes, which are myelin-producing cells and the presumed target of the autoimmune reaction in MS (Ahlqvist 2005). Furthermore, HHV-6A infected oligodendrocyte progenitor cells do not migrate properly, which affects the myelin repair process in the brain (Campbell 2017). Supernatants from HHV-6A infected cells induce immunogenic programmed cell death in oligodendrocytes (Kong 2003) and HHV-6A has been found in the plasma of MS patients during relapse (Alvarez-Lafuente 2004). Finally, HHV-6A can activate EBV proteins that are important for EBV immortalization of B-cells (Flamand 1993).
One hypothesis is that HHV-6A infection and EBV activation in the brain of MS patients leads to intrathecal B-cell transformation, and that they both induce expression of endogenous retrovirus. These virally induced T-cell responses might secondarily lead to local autoimmune phenomena (Fierz 2017).
The authors conclude that there is strong serological data supporting a role for HHV-6A in MS etiology, but that causality remains to be proven.
“We hope that this publication can motivate researcher and funding agents to continue explore the biology, pathology and epidemiology of these viruses and lead us to understand how they might contribute to autoimmune disease,” said Dr. Fogdell-Hahn.
A short summery of the article can be found on youtube at this link:
Read the full paper: Engdahl 2019

