Several mechanisms have been identified by which HHV-6A/B infection of the brain could contribute to the pathogenesis of several important neurological diseases in some people. Previously, investigators at The Sapienza University of Rome demonstrated that infection of HSB2 and Molt-3 cells, or human monocytes, dysregulated autophagy and activated the unfolded protein response (Romeo 2020). Now they report on what happens when HHV-6A infects astrocytoma cells.
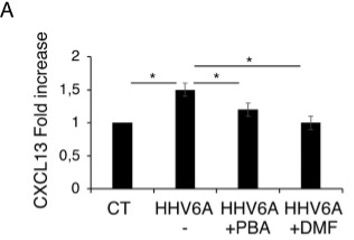
Figure 1 – CXCL13 increase in HHV-6A cells and effects of PBA and DMF. *indicates P-value <0.05
Infection of these cells by HHV-6A reduces autophagy, increases production of reactive oxygen species (ROS), induces endoplasmic reticulum (ER) stress, promotes the production and release of IL-6 and IL-1ß by the infected cells and activates three important pathways of inflammation: STAT3, NF-κB, and mTOR. It also increases the production of CXCL13, a B cell attracting chemokine (Figure 1). B cell migration into the brain plays an important role in the pathogenesis of multiple sclerosis and Alzheimer’s disease.
Finally, HHV-6A infection of these cells increases production of cathepsin S, leading to the degradation of myelin basic protein (MBP). All of these consequences of HHV-6A infection could plausibly contribute to the pathogenesis of neurological diseases.
Of potential importance, two molecules—dimethyl fumarate (DMF) and sodium 4-phenylbuyrate (4-PBA)—reduce all of these potentially destructive processes. These molecules are known to counteract ER stress, inflammation and oxidative stress.
The relevance of these in vitro experiments for HHV-6A infection of the brain in vivo, and for potential treatments of people with neurological diseases, remains to be determined.
Read the full article: Romeo 2022