Plaques of amyloid-ß (Aß), and tangles of tau protein, are central features of the pathology of Alzheimer’s disease (AD). Furthermore, Aß and tau both are neurotoxic. In recent years, it has become clear that neuroinflammation also is central to the pathology of AD. But what generates the neuroinflammation?
Studies over the past 20 years have suggested that persistent herpesvirus infection of the brain may be one cause of neuroinflammation in AD. Most of the evidence involves herpes simplex virus-1 (Itzhaki 2020), although some studies have incriminated other herpesviruses, particularly Epstein-Barr virus, varicella-zoster virus, HHV-6A/B and HHV-7. The evidence for HHV-6A/B and HHV-7, however, has not been widely replicated, as reviewed elsewhere (Komaroff 2020).
In addition, it has been reported that Aß is an antimicrobial peptide that is upregulated in response to infection and inhibits acute infections (Eimer 2018). Thus, a chronic viral infection (like HHV-6A/B or HHV-7) could trigger long-term, low-level production of Aß: in short, the production of Aß may protect the brain from an acute infection but Aß is itself neurotoxic and its ongoing chronic production may therefore lead to neurodegeneration. Thus, the hypothesis: herpesvirus infection of the brain could lead to neuroinflammation, leading to chronic production of Aß, which then leads to neurodegeneration.
Since murine roseolovirus (MRV) is highly related to HHV-6A/B, investigators at Washington University examined this hypothesis in mice. They inoculated very young wild-type mice and mice engineered to overproduce Aß with MRV, both peripherally and in the brain.
Even peripheral inoculation subsequently resulted in chronic brain infection. Acute infection of the brain led to activation of glial cells and the production of tau. As previously reported, experiments in vitro found that Aß interacted with viral particles and reduced viral replication as well as MRV-associated disease.
However, when the mice were sacrificed at 6 months, there was no evidence of increased deposition of Aß. Moreover, they did not find that 6-week old mice had increase Aß deposition. Thus, the first steps of the hypothesis that MRV infects the brain and induces neuroinflammation were supported. However, the critical last step—the increased or accelerated deposition of Aß—was not.
The investigators then examined human tissue: brains from 350 people with AD and 31 healthy control subjects. Using RNA-seq to screen for a very large number of microbes, including HHV-6A/B and HHV-7, they found no evidence of increased microbial sequences in people with AD than in controls, as shown in Figure 1.
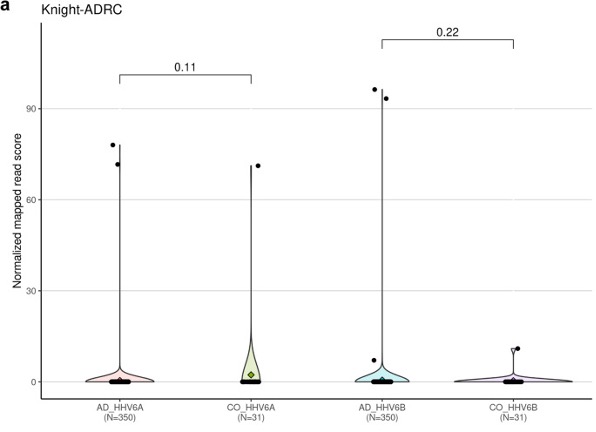
Figure 1 – HHV-6A and HHV-6B viral abundance between Alzheimer’s disease (AD) and controls (CO) and corresponding P-values.
Thus, the mouse roseolovirus studies provide some support for the possibility that roseolovirus infection could be one trigger of AD, but fail to provide support for the critical last step in the hypothesis: the increased deposition of Aß.
The investigators acknowledge that it is possible that a larger inoculum of MRV might have generated more pathology. The authors discussed that it is possible that additional stressors or immune deficiency that might increase reactivation or viral load could be important factors required for MRV to increase Aß deposition. Another consideration is that although they inoculated the mice very early in life (the time when humans typically become infected with roseoloviruses), they sacrificed the animals at 6 months—which is 30-45% of the animals’ lifespan. AD typically becomes manifest in humans after about 75% of the lifespan. Might sacrificing the animals at a later time have revealed increased deposition of Aß?
The authors conclude that their findings “do not support or refute the antimicrobial protection hypothesis”. While their studies of human brains do not indicate that infection with human roseoloviruses is an important contributor to the pathogenesis of AD at a population level, their studies do provide an in vivo system to study how specific factors, such as immune response and stress, might contribute to neuroinflammation and Aß deposition during roseolovirus infection.
Read the full article: Bigley 2022