Previous research has suggested that HHV-6A/B may both trigger some cases of autoimmune thyroiditis (AIT) (Caselli 2012) (Sultanova 2017). Viral DNA has been found much more often in thyroid tissue from AIT than tissue from healthy control subjects, and viral mRNA indicative of active infection also has been found more frequently in AIT tissue.
A team from Riga Stradins University in Latvia pursued one possible mechanism by which the viruses might trigger autoimmunity. Both viruses contain two genes, U12 and U51, that encode viral chemokine receptors that are putative homologues of human G-protein-coupled receptors (GPCRs). These viral proteins are expressed on the surface of both epithelial and blood mononuclear cells. Thus, if the viruses infected thyroid cells, these viral proteins could be recognized and attacked by the immune response, injuring the thyroid cells. In short, the immune attack would involve “autoantibodies” directed at viral epitopes but injuring “self” tissue.
The investigators created a group of synthetic peptides derived from U12 and U51 and then conducted multiplex assays to detect IgG and IgM antibodies directed at these peptides in 79 patients with autoimmune thyroiditis and 32 healthy control subjects. In all 79 patients, HHV-6 DNA had been demonstrated in thyroid tissue—so the study group was a subset (of uncertain size) of all patients with AIT.
IgG antibodies against two of the synthetic peptides were found much more frequently in AIT patients, although IgG against other synthetic peptides was not. IgM antibodies against 3 of 10 peptides tested were found much more frequently in AIT patients. In AIT patients with IgG against the synthetic viral peptides, there were higher levels of two thyroid autoantibodies: thyroid peroxidase antibodies (TPO) and thyroglobulin antibodies (TG), as shown in Figure 1.
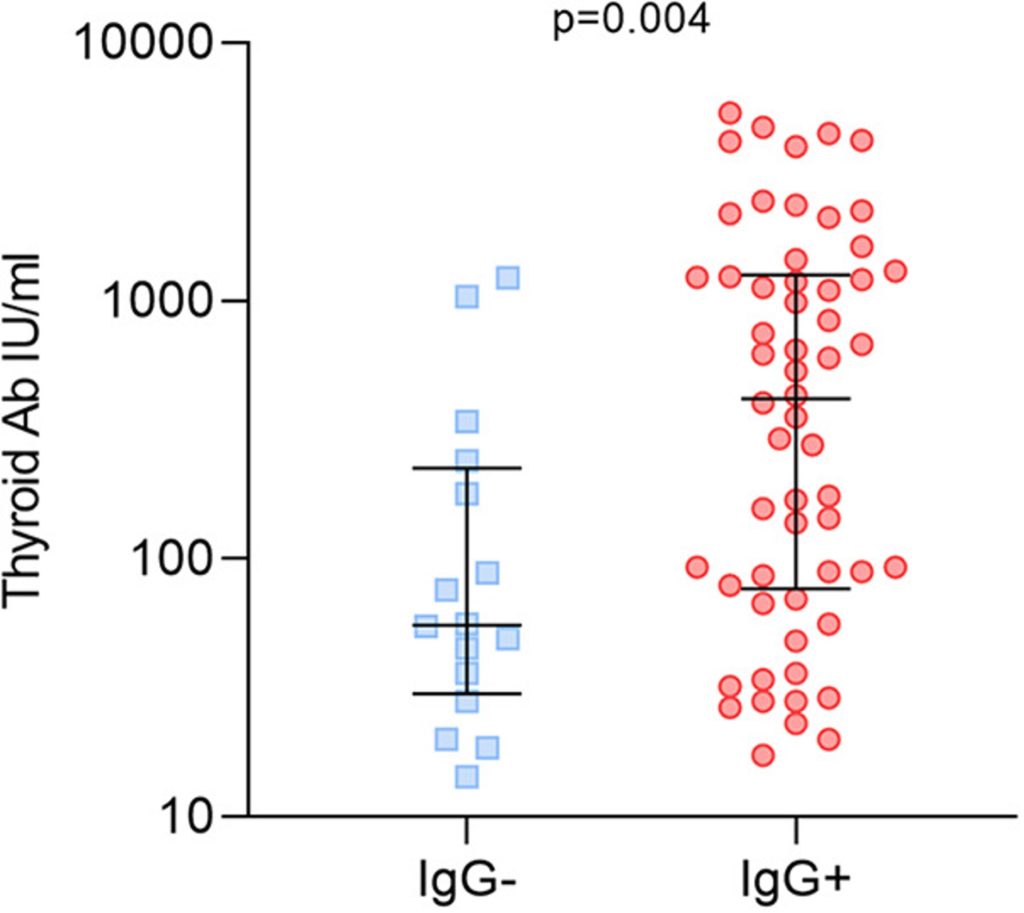
Figure 1 – Patients with acute autoimmune thyroiditis positive and negative for IgG antibodies to HHV-6 peptides and thyroid autoantibodies levels.
This study provides some evidence that one mechanism by which infection of thyroid tissue by HHV-6A/B could provoke an immune response that damages thyroid tissue: viral epitopes displayed on the surface of thyroid cells could be the target of tissue-damaging antibodies.
Read the full study here: Sultanova 2022