Individuals carrying this polymorphism had a five-fold increased risk of depression and were more likely to have a family member with major depression.
Major depressive disorder (MDD) is one of the world’s most important health problems, significantly impairing the quality of life and leading, through suicide, to a large number of early deaths. MDD affects 15-18% of the world’s population at some point in their lives. MDD often is found in multiple family members: its heritability is between 30-50%. However, human genomic studies have found few robust linkages with the phenotype of depression, and those linkages that are robust have had only a small predictive value.
Human genes are not the only genes passed by parents to their children. Parents can pass viral genes to their children, in several ways. Most commonly, exogenous virus can be passed after birth to the infant from either parent, usually via saliva. Less often, in 1-3% of births, a parent passes on endogenous inherited chromosomally integrated HHV-6 (iciHHV6), which is capable of reactivation. (Hall 2008; Hall 2010)
Previously, in 2020, a team from The Jikei University School of Medicine in Tokyo, led by Kazuhiro Kondo, reported that SITH-1, an exogenous HHV-6B latent protein specifically expressed in astrocytes, activated the hypothalamic-pituitary-adrenal (HPA) axis and caused depressive symptoms in mice. Upregulation of the HPA axis is found often in MDD.
The team then measured levels of antibodies to SITH-1 in people with major depression, and in healthy control subjects. Levels were significantly higher in the former group (P = 1.78 X 10-15); detectable antibody was found in 79.8% and 24.4%, of the two groups, respectively (odds ratio was 12.2.) (Kobayashi 2020)
A new report from the team pursues this link further. They sequenced virus from different human saliva specimens in 46 people with MDD, 31 people with other psychiatric disorders, and 77 normal control subjects. Those with MDD were similar to the normal controls with regard to age, gender, body weight and the amount of HHV-6B DNA in saliva.
The team evaluated three regions in the latency gene encoding region (R1, R2 and R3), each of which had various polymorphisms. The R1 region is upstream of exon 1 of the mRNA of SITH-1 protein, and partially overlaps with exon 1.
The R1A region was found to contain different numbers of repeat sequences in the viruses isolated from the saliva of different people. The number of repeat sequences in HHV-6B was found to have an inverse correlation with the expression of SITH-1 in astrocyte cell line U373. Specifically, isolates with relatively low numbers of repeat sequences (≤ 17) had significantly higher levels of SITH-1 expression than isolates with > 17 repeat sequences.
Among the human subjects studied, people infected with HHV-6B containing relatively few repeat sequences were much more likely to have MDD: 67.9% of people with MDD had HHV-6B with ≤ 17 repeat sequences, whereas that was true of only 29% of normal control subjects (odds ratio of 5.28). People with MDD also had significantly higher levels of antibodies to SITH-1, as shown in Figure 1.
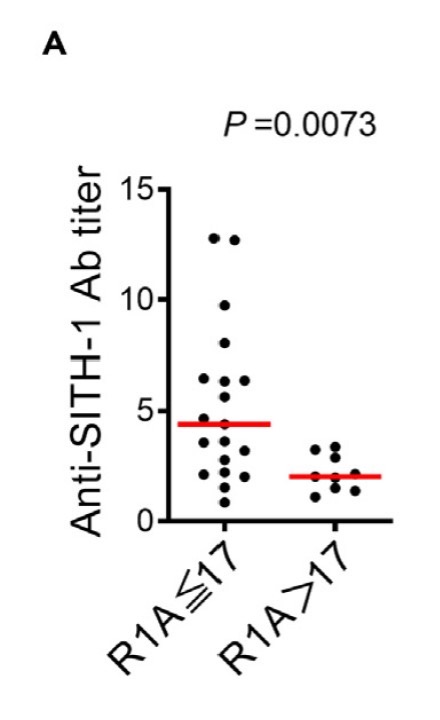
Figure 1. Difference in Anti-SITH-1 antibody titers in MDD patients depending on whether number of R1A elements is 17 or less or greater than 17. The red bars represent median values.
The symptoms of MDD most strongly associated with fewer R1A repeat sequences—as determined by a validated questionnaire for depression—were suicidality, psychomotor retardation and gastrointestinal somatic symptoms. These symptoms are reportedly associated with enhancement of the HPA axis.
The team then examined the correlation of the number of direct repeats with a reported history of other family members who also suffered from MDD. Among people with ≤ 17 repeat sequences, 47% reported another family member who suffered from MDD. In contrast, among people with > 17 repeat sequences, 0% reported another family member with MDD (p=0.03).
The investigators did not determine whether some of the patients might have iciHHV-6. With iciHHV-6, the virus is not only passed through the germ line: in addition, reactivated iciHHV-6 can also pass transplacentally from an iciHHV-6 mother to the fetus during pregnancy.
This provocative report adds strength to the hypothesis that an HHV-6B latent protein specifically expressed in astrocytes—previously shown capable of activating the hypothalamic-pituitary-adrenal (HPA) axis and causing depressive symptoms in mice—may also be linked to MDD in humans.
An additional and remarkable hypothesis suggested by this report is that MDD may “run in families”, in part, because exogenous HHV-6B (or even endogenous) viruses containing relatively few R1A repeat sequences have been passed from parents to offspring at birth or in early infancy. The study was small needs to be confirmed by a much larger study.
Read the full article: Kobayashi 2024

