Israeli study of 20 pediatric cases occurring in 2021 found more evidence for HHV-6 than for other viruses, including AAV2 and SARS-CoV-2.
Following reports elsewhere in the world of cases of acute hepatitis of unknown origin in children during the COVID-19 pandemic, a national registry was established by the Israeli Ministry of Health. Results from 20 cases occurring in 2021 were systematically captured, although no formal prospective study with protocol-directed testing was conducted.
Many of the children had severe hepatitis. The median lab values were AST 1334 (IU/L), ALT 1240 (IU/L), total bilirubin 4.5 (mg/dL), and INR 1.14 (Table 1). Eight children were treated with steroids due to suspicion of autoimmune hepatitis and impending liver failure. Two children underwent liver transplantation.
The following studies were performed:
- COVID-19 status was determined using tests for viral nucleic acid, viral antigen or positive serology, with any positive result taken to mean the subject had been exposed.
- In all cases, there was no evidence of infection with the common hepatitis viruses (A-E), HIV, EBV, or CMV.
- Qualitative real-time PCR (RT-PCR) of whole blood was used for the detection of human adenovirus, adeno-associated virus-2 (AAV2), human herpes viruses 1 and 2, and human herpes virus 6 (HHV-6)—not distinguishing between HHV-6A and HHV-6B.
- HHV6 serology was performed using indirect fluorescent antibody (IFA) IgG and IgM assays.
- Qualitative RT-PCR in plasma and in stool was used to detect adenovirus, enterovirus, norovirus and rotavirus.
- Respiratory viruses (adenovirus, influenza A virus, influenza B virus, metapneumovirus, parainfluenza virus, respiratory syncytial virus, human rhinovirus) were detected in nasopharyngeal swabs.
- No data was provided regarding the sensitivity and specificity of the assays used.
- No control or comparison patient groups were tested with the same assays.
As shown in the Table, below:
- In contrast to studies in other countries, prevalence of both adenovirus and AAV2 was low.
- 68% of the children had evidence of past exposure to SARS-CoV-2, although without a control/comparison group it is unclear how this compared to children without acute hepatitis. Also, the authors did not determine whether the SARS-CoV-2 infections were recent or active at the time of the hepatitis. Patients counted as “positives” included those with antibodies indicating past exposure.
- Overall, blood samples from eleven children (58%) were HHV6 positive: six were just PCR-positive, three just had high anti-HHV6 IgG levels, and two were both PCR positive and had high levels of IgG. In one patient, evidence of both HHV6 and adenovirus was found.
- Most of the children were older than two years old (31/39 in total; 9/11 of the patients with HHV6). Since this is an age at which most children are likely to have already been infected with the virus, some of these cases may have involved reactivation rather than primary infection.
- Only one of the patients was negative to all tested viruses.
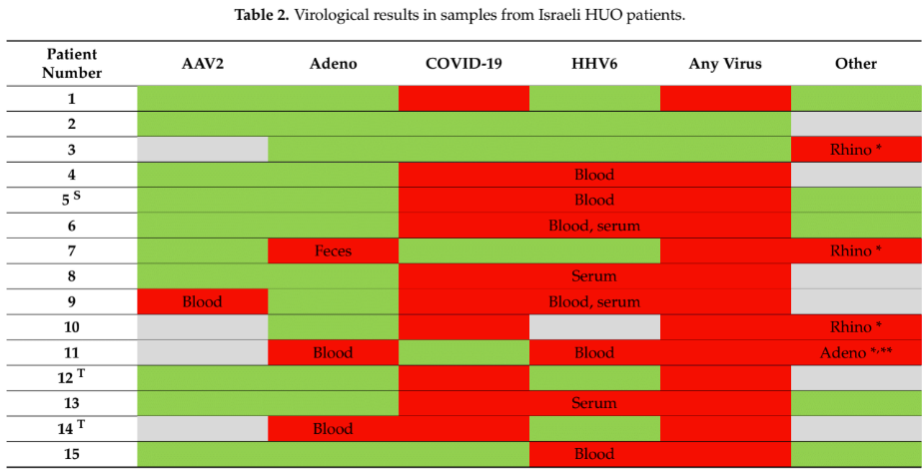

Unlike several other studies of hepatitis of unknown origin occurring in young children in the 2020-2022, this study found less evidence that AAV2 or adenovirus might be responsible for the hepatitis, and more evidence that HHV-6 (unclear whether -6A or -6B) was present.
However, the uncertainty around the sensitivity and specificity of the assays used, and the lack of control/comparison groups tested with the same assays, makes these data hard to interpret.
Read the full text: Shteyer 2024

