1.1% of 72,423 HCT patients developed encephalitis with an all-cause mortality rate of 60%.
A systematic review of viral encephalitis in hematopoietic cell transplant (HCT) patients, led by Danielle Zerr and Joshua Hill of Seattle Children’s Hospital and the University of Washington, includes 68 studies involving 72,423 patients following various forms of HCT.
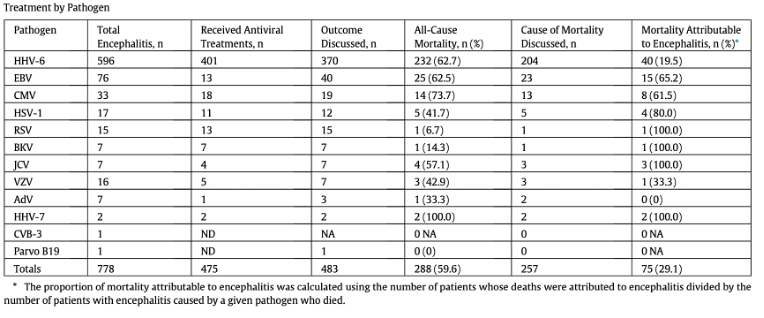
Encephalitis occurred in 778 (1.1%) of all cases of HCT. As shown in the Table below, HHV-6 was by far the most common cause of encephalitis, causing 596 (77%) of cases; in contrast, 76 (9.8%) were due to EBV and 33 (4.2%) were due to CMV.
The rate of encephalitis was much higher (6%) with cord blood transplants (CBT) as compared to 1.5% with non-CBT allogenic transplants and 1.7% with autologous transplants. Moreover, almost all of the CBT encephalitis cases (96%) were attributed to HHV-6.
Encephalitis that occurred within the first 100 days following transplantation was extremely likely to be caused by HHV-6: encephalitis due to other viruses was more likely to be occur after the first 100 days.
All-cause mortality was close to 60% in patients with encephalitis following HCT, although the encephalitis was responsible for the mortality in only about half (29%) of the patients who died. Encephalitis caused by EBV and CMV, while much less common than encephalitis caused by HHV-6, was more likely to be the cause of death, as seen in the Table, above.
Of the studies reporting data on symptoms, 14.1% presented with seizures, 10.4% with rash and 3.1% with hyponatremia and syndrome of inappropriate antidiuretic hormone (SIADH) secretion, virtually all of which were associated with HHV-6. Two thirds of those with neuroimaging showed abnormalities.
The authors note that given the high level of morbidity and mortality related to HHV-6 encephalitis, antiviral therapy should be strongly considered. They point out that while low dose (90 mg/kg) foscarnet prophylaxis has been found to reduce symptom severity and rates of sequelae, it has not been found to lower the incidence of HHV-6 encephalitis (Ogata 2018). However a study at University of Michigan found that low-dose foscarnet prophylaxis improved engraftment rates and overall survival in cord blood transplant patients (Jurdi 2020). A randomized controlled trial of brincidofovir reduced the incidence and magnitude of HHV-6B reactivation after allogenic HCT (Hill 2020) and is currently in clinical trials in Japan.
This review highlights the importance of HHV-6 in causing encephalitis following HCT, particularly following CBT and in patients with relatively early onset of encephalitis following HCT, as well as the high mortality rate in these patients. The review also highlights the need for more studies of strategies to prevent and treat the reactivation of HHV-6 in people undergoing HCT.
See the full article: Toomey 2023
Source: Transplantation and Cellular Therapy, Toomey 2023

