Findings suggest role in evading host immune defenses.
The U20 proteins of the roseoloviruses are membrane glycoproteins possessing class I major histocompatibility complex (MHC)-like folds. They are generated by a block of genes unique to the roseoloviruses. These genes are expressed with early kinetics, dispensable for viral growth and thought to play a role in evading host defenses. The U20 proteins from HHV-6A and -6B share 92% homology, but past research has ascribed to them different roles in immune evasion: HHV6A U20 downregulates NKG2D ligands, impairing recognition of infected cells by NK cells, while HHV6B U20 inhibits tumor necrosis factor alpha (TNF-a)-induced apoptosis during nonproductive infection.
A team from the Medical College of Wisconsin led by Amy Hudson performed cell biological and biochemical characterization of the trafficking, glycosylation, and posttranslational modifications occurring with HHV6B U20. They find that HHV-6B U20 traffics very slowly through the secretory system, undergoes several posttranslational modifications to its N-linked glycans that are characteristic of surface-expressed glycoproteins, eventually reaches the cell surface and then becomes internalized.
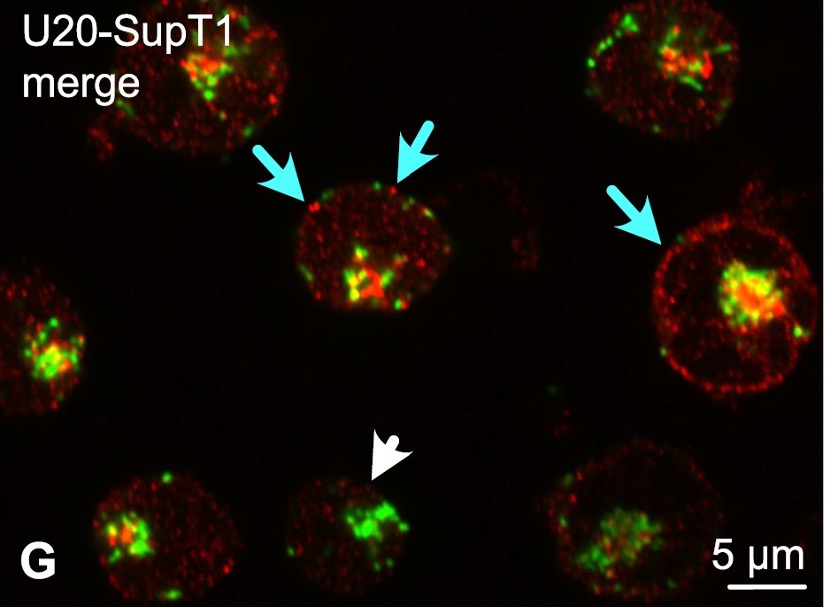
Figure 1: Confocal microscopic colocalization of HHV-6B U20 (in red) and lamp1 (in green), a marker for the late endosomal-lysosomal pathway, in SupT1 cells expressing U20. White arrowhead indicates a cell that does not express U20. Blue arrows indicate U20 that appears at or beneath the cell surface. HHV6B U20 did not colocalize with lamp2; instead, it localized at or beneath the cell surface.
These careful studies may help shed light on the function of the HHV-6B U20 protein, particularly its possible role in immune evasion.
Read the full article: Schneider 2023