Transposable elements in aging brains were linked to herpesvirus RNA, predominantly HHV-6A, HHV-6B and HHV-7.
A team lead by Cleveland Clinic’s Genome Center's Director Feixiong Cheng, PhD, reported evidence associating molecular, clinical and neuropathological features of Alzheimer’s Disease (AD) with human herpesvirus infection. Dr. Cheng’s hypothesis was that latent herpesvirus infections could trigger AD by directly activating the transposable elements which his laboratory and previously connected to disease progression in aging brains. His group reported last year that there is widespread transposable element (TE) dysregulation in human aging brains with AD (Feng 2024).
Transposable elements, often called "jumping genes," are mobile DNA sequences capable of changing their position within a genome. This movement can alter genetic identity, create or reverse mutations, and influence genome size. Discovered by Barbara McClintock in the 1940s (earning her a Nobel Prize in 1983), TEs are now recognized as major drivers of genetic diversity and evolution
Cheng's group found that TE dysregulation correlated with HHV positive human Alzheimer’s brains, including astrocyte specific upregulation of the LINE1 (long interspersed nuclear element 1) subfamily TEs. The LINE1 dysregulation could be partially reversed using HHV antivirals valacyclovir and acyclovir in their HSV-1 infected human brain organoid model.
The group analyzed bulk RNA-seq data in two large human brain biobanks: the Religious Orders Study and Rush Memory and aging project (ROS/MAP) and the Mount Sinai Brain Bank (MSGG) as well as single-cell RNA sequencing data from HHV infected forebrain organoids to investigate HHV-infection associated TE dysregulation.
Between the two biobanks they studied 393 AD cases and 194 cognitively healthy controls. They found widespread TE activation in the HHV positive AD brains, and significantly positive associations of HHV RNA abundance with the APOE4 genotype, Braak staging score and CERAD (Consortium to Establish a Registry for Alzheimer’s Disease) score. The APOE4 genotype, specifically the APOE4 allele (or ε4 allele), is a genetic variant associated with an increased risk of AD. The Braak staging score is a semiquantitative system used to classify the severity and distribution of specific brain pathologies in AD. The CERAD scores is semiquantitative neuropathological measure used to assess the density of neuritic plaques in an AD brain.
The sequential process suggested by the authors is that the process begins as herpesviruses becomes more active as our immune systems falter as natural consequence of aging. The herpesviruses then activate transposable elements such as those in the LINE1 subfamily, which in turn disrupts key genetic processes in the brain resulting in the accumulation of tau and associated inflammation and neurodegeneration.
Although the authors chose HSV-1 for their organoid model, the predominant HHV RNAs found in brain cells were HHV-6A, HHV-6B and HHV-7.
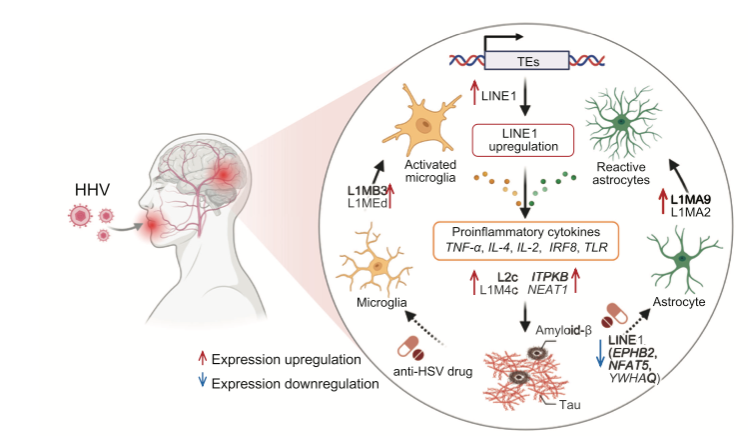
Proposed transposable element upregulation-associated AD-like mechanisms in human AD brains.
The investigators utilized real-world data from the Optum database (604,026 records) to demonstrate that treatment with valacyclovir (HR =.803) and acyclovir (HR= 0.87) significantly reduced the incidence of AD, particularly in women and older adults.
Read the full paper: Feng 2025