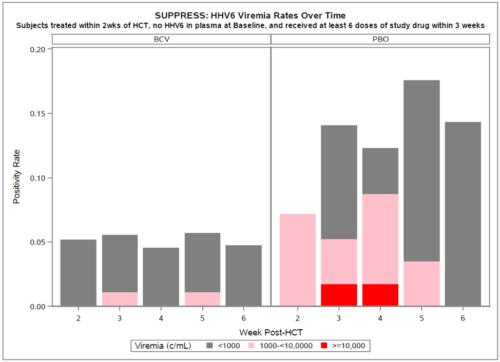
An abstract at the Transplantation & Cellular Therapy Meeting in Houston showed that only 2% of 92 patients treated with oral brincidofovir developed high level reactivation compared to 11% of 61 patients taking the placebo. The results came from an analysis of stored samples from their previous Phase III SUPPRESS trial for CMV prophylaxis.
 Chimerix’s Phase III trial for cytomegalovirus failed. Although oral brincidofovir reduced CMV viremia during the first 14 weeks, CMV infections bounced back during the 10-week observation period that followed, and there was a higher mortality rate in the drug group.
Chimerix’s Phase III trial for cytomegalovirus failed. Although oral brincidofovir reduced CMV viremia during the first 14 weeks, CMV infections bounced back during the 10-week observation period that followed, and there was a higher mortality rate in the drug group.
Analysts blamed the higher death rate on the inability of participating physicians to differentiate between bleeding (a side effect of the drug) and acute GVHD. Instead of adjusting the dose as was the protocol, physicians may have administered unnecessary immunosuppression, potentially increasing mortality. Another probably reason for failure of the Phase III trial was the company’s error in not following the same protocol used in their successful Phase II trial. Chimerix executives erred by starting the drug two weeks earlier in the Phase III trial -- at a time when the gut was still inflamed. This is believed to have increased the rate of bleeding.
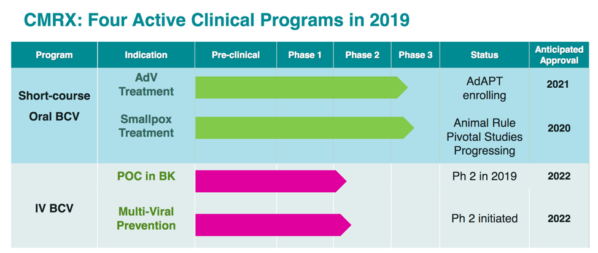
Animal studies have shown that the IV form of brincidofovir has better penetration to the brain and organs without the gastrointestinal toxicity associated with the oral version. For these reasons, IV brincidofovir may be better suited for the prevention of HHV-6 encephalitis. IV brincidofovir is currently in Phase II trials for multivirus prevention. Oral brincidofovir is largely absorbed in the gut.
Chimerix is hoping to get FDA approval for brincidofovir treatment of both adenovirus and smallpox, but they are in a difficult financial situation currently with their stock trading at cash value.
Read the Chimerix press release.

