Investigators in Spain and Italy attempted to reduce the rate of acute GVHD in pediatric transplant patients by infusing manipulated stem cells. Not only was there no reduction in expected acute GVHD, the patients experienced an unusually high rate of HHV-6 disease, far higher than in previous haploidentical studies. The authors suspect that the high percentage of HHV-6 infected CD4+ T cells and low percentage of NK cells may be to blame.
The studies were conducted at two pediatric transplant centers in Spain and one in Italy. The Spanish cohort of 25 patients received grafts depleted of naïve T (CD45RA+) cells (preserving memory T cells to fight against infection). The Italian cohort received CD34+ cells plus regulatory T cell and conventional T cells (Treg/Tcon).
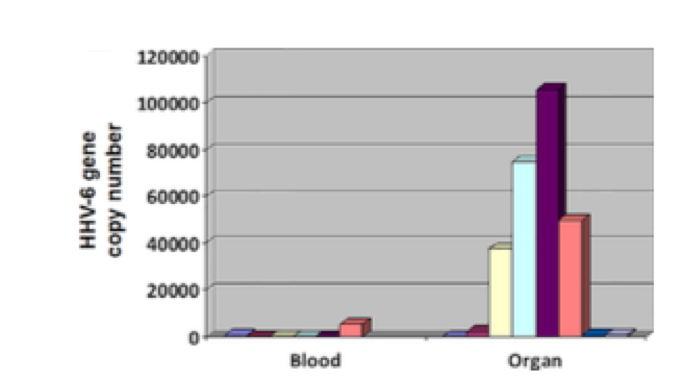
Patients developed organ disease as well as encephalitis, with HHV-6B DNA levels far higher in the BAL, liver, GI tract an brain than in in the blood. Source: Biology of Blood and Marrow Transplantation
The CD45RA+ depleted patients experienced a 32% rate of encephalitis while 6/13 of the Spanish cohort receiving the Treg/Tcon protocol developed HHV-6 organ disease (hepatitis, pneumonia, gastroenteritis), with viral load in the organ biopsy samples far higher than levels of HHV-6 DNA in the blood. Between the two groups, there were no statistically significant differences in aGVHD/cGVHD incidence, overall survival, immune reconstitution or transplant-related mortality, or relapse. The HHV-6 viral load in the organ biopsies was 2268 copies/mL vs. a median viral load of 95 copies/mL in the blood. A similar phenomenon has been reported for solid organ transplantation (Ogata 2018a).
To confirm that NK cells are critical for controlling HHV-6 infection, the authors isolated T cells from the patients. Treatment with escalating doses of ganciclovir decreased the viral load in vitro but did not eliminate the virus. In contrast, when the CD4+ cells were co-cultured with CD56+ NK cells, they were able to eliminate the virus at a ratio of 2:1 (NK:CD4+) or greater. Since HHV-6B infects CD4+ cells and not CD8+ cells, HHV-6 was detected in only CD4+ cells.
Since all patients in the study responded to antivirals, the authors suggest that routine surveillance of HHV-6 would be useful to diagnose and treat early HHV-6 infection. (Guidelines from the 7th European Conference on Infections in Leukemia did not recommend routine surveillance of HHV-6.) They also propose enrichment of NK cells and a search for more effective antivirals for patients undergoing these transplant protocols.
Although all 9 patients responded to antiviral treatment, 2 patients suffered long-term neurological sequelae that persisted for 12 and 22 months, respectively.
Patients were treated with foscarnet or ganciclovir when their HHV-6 viral load reached >1000 copies/mL in the Italian cohort, whereas patients from the Spanish cohort received the same treatment if they had 3 consecutive positive qualitative PCR results. PCR DNA testing was performed twice a week routinely from conditioning to CD4+ recovery for both cohorts. Only the Italian cohort underwent screening for iciHHV-6.
Both cohorts achieved engraftment within 20 days (median 15 days for Treg/Tcon grafts and 10 days for CD45RA-depleted grafts), and all patients showed full donor chimerism by day +30. All 38 patients developed HHV-6 infection, and onset of HHV-6 infection was earlier in the Treg/Tcon group (median day +9, range 5-16) compared to the CD45RA-depleted cohort (median day +13, range 13-17).
The authors suggest that HHV-6 reactivation could have played a role in triggering aGVHD in their study, which is possible as the grafts used in this study are rich in CD4+ cells, the preferred site of latency for HHV-6B.
The role of HHV-6 in the setting of stem cell transplantation differs greatly depending on the type of graft and conditioning. Past studies have shown that cord blood transplantation is associated with the greatest risk of developing HHV-6 encephalitis (approximately 8% incidence of HHV-6 encephalitis compared to 0.5-2.3% in non-CBT; Ogata 2018a).
The Spanish group published a separate paper for their results (Sisinni 2018). They noted that pre-engraftment syndrome occurred before the patients developed HHV-6 encephalitis, a phenomenon also noted previously in Japan (Morita-Hoshi 2010, Ogata 2018a). Click here to read our past coverage of this study.
Read the full paper: Perruccio 2018.
| Variables | Sisinni et al. 2018 | Tripplett et al. 2018 |
| Patients (n) | n=25 | n=26 |
| Age | 12 (2-17) | 10.5 (0.6-20.7) |
| Underlying disease | B-ALL: 10 T-ALL: 6 AML: 7 Biphenotypic: 2 |
B-ALL: 8 T-ALL: 3 AML: 11 Mixed lineage leukemia: 2 Myelodysplastic syndrome: 1 Lymphoma: 1 |
| Haplo-donor | Mother: 16 Father: 8 Brother: 1 |
Mother: 17 Father: 7 Sibling: 2 |
| GVHD Prophylaxis | Cyclosporine: 3 Cyclosporine+Methotrexate: 1 MMF: 21 |
MMF: 17 Sirolimus: 9 |
| Antiviral prophylaxis | Acyclovir | Acyclovir |
| Infusion data | 1st product:
CD34+: 6.29e6/kg (4.04-18.1) 2nd product: CD45RA+: 5.3e3/kg (0-14.6)
|
CD34+: 15.6e6/kg (2.9-67.5) CD3+: 80e6/kg (16.1-528.6)CD3+/CD45RA+: 11e3/kg (2-130)CD3-/CD56+ (NK): 14.4e6/kg (1.5-422.5) |
| Results | Sisinni et al. 2018 | Tripplett et al. 2018 |
| Neutrophil engraftment | 10 days (8-18) | |
| Platelet engraftment | 14 days (10-151) | |
| T-cell reconstitution (ALC = absolute lymphocyte count) | Median ALC: 1,200 cells/µL (day +15) Median ALC: 2,200 cells/µL (day +90) |
Median T cell count: 550 cells/µL (day +30)
CD4+: 140/µL (day +30) B cell: ~80/µL (day +90) |
| GVHD | aGVHD (n=13) Grade 1: n=5 Grade 2: n=2 Grade 3-4: n=6 Grade 2-4: 39% (cumulative) Grade 3-4: 33% (cumulative)cGVHD (n=3) Moderate in 2, mild in 1 (22% cumulative) |
aGVHD (n=6; 23.1%) Grade 3-4: n=6, 23.1% |
| HHV-6 Encephalitis | n=8 (34% cumulative) Onset 35 days (13-56) |
None reported |
| Infections | AdV/BK: n=1 Parvovirus: n=1 Influenza A: n=1 CMV: n=8 (treated; no CMV disease) |
Viremia: n= 9 (35%) Prolonged viremia: n=1 CMV: n=3 (treated out of 5 patients) EBV: n=5 (19%) AdV: n=1 (4%; viral load <threshold of detection)88% of patients received brief GCV/foscarnet therapy (median duration 14 days, range 3-141)), with 5 (19%) receiving 4 weeks or more: “CD45RAdep recipients received short-course GCV in the early post-transplant period despite the absence of CMV for preemptive treatment of HHV6 in the setting of fever and rash.” |
| Transplant-related mortality | 16% at day +100 22% at 30 months post-transplant |
15.4% at day +180 |
| Relapse | n=3 (12%) 20% at 30 months (cumulative) |
|
| Survival | N=15 (60%) at median 365 days (8-898) Overall survival (OS): 58% at 30 months follow-up |