A new point-of-care assay from bioMérieux can simultaneously and rapidly detect 14 pathogens typically found in encephalitis. The machine is designed to be at the clinic or in the emergency room and can be operated by unskilled technicians. In a study of 1,560 immunocompetent patient samples, a total of 1.35% were positive for HHV-6, or about twice the expected rate of 0.8% found with the inherited chromosomally integrated HHV-6.
The BioFire FilmArray Meningitis/Encephalitis (ME) Panel is designed to run on cerebrospinal fluid and identifies HHV-6A & HHV-6B, as well as CMV, and HSV-1 and -2, e coli K1, haemophilus influenza, listeria monocytogenes, neisseria meningitides, streptococcus pneumonia and streptococcus agalactiae, human parechovirus, varicella virus, cryptococcus neoformans and cryptococcus gatti.
The panel was used to evaluate cerebrospinal fluid (CSF) samples from patients at 11 distinct U.S. sites, 59% of whom were adults 18 years of age or older.
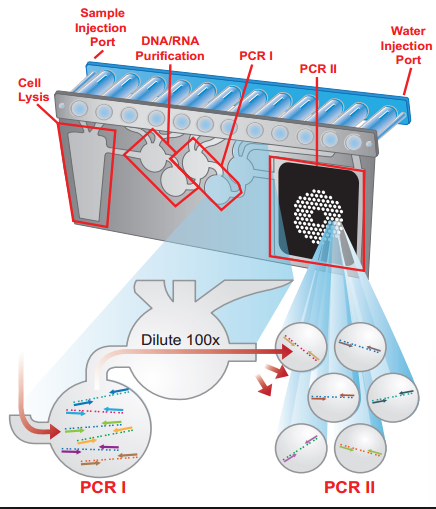
FilmArray multiplex PCR system by bioMerieux
Overall, the assay had a sensitivity or positive percentage of agreement of 100% for 9 of the 14 bacterial, yeast, and viral agents. HHV-6A/B had an agreement percentage of 85.7%. Taking into account all 21,840 individual analyte tests performed by the FilmArray on the 1,560 samples, the percentage of agreement between FilmArray and the comparator testing was 99.8%, with 43 false-positives and 6 false-negatives. The comparator testing had a sensitivity threshold of 1000 copies/mL. The simplicity of the testing process, the range of common ME pathogens it detects, and the ability to quickly test the sample on site suggest that the FilmArray could be a valuable clinical tool in a variety of settings.
A total of 21 of the 1,560 samples were positive for HHV-6, and 17 of the 21 positive samples were from children under 18, leading to a rate of HHV-6 positivity in pediatric patients of 2.7%. The assay identified 4 false-positives and 3 false-negatives for HHV-6. In most cases, HHV-6 infection was not the final diagnosis, and in many, another infectious agent was found in addition to HHV-6.
CiHHV-6 is found in approximately 0.8% of the population in the U.S., and the virus is typically found in the CSF of ciHHV6 individuals who are acutely ill (although ciHHV6 could fall below the threshold of detection in a convalescent patient). CiHHV-6 can reactivate and cause neurological symptoms (Montoya 2012), but it may also be a bystander in other cases.
HHV-6 has been found in the CSF of young children with encephalitis and febrile convulsions at higher rates using assays with similar sensitivity (Yavarian 2014, Parisi 2016). Even in cases in which HHV-6 is identified as the causative agent of encephalitis, detection of the virus in the CSF is difficult, as it is only found transiently in the cerebrospinal fluid.
Read the full paper here: Leber 2016, and visit our page on HHV-6 and encephalitis to learn more about the roles of HHV-6A and B in the development of encephalitis in both immunocompromised and immunocompetent individuals.

