Bhupesh Prusty and Thomas Rudel of University of Wuerzburg, Germany, in collaboration with Dr. Yasuko Mori of Japan, have shed new light on the long-standing mystery of HHV-6 cell tropsim. CD46 and CD134 serve as essential cell surface receptors for HHV-6A and HHV-6B, respectively, facilitating viral binding to human cells through direct interaction with different viral glycoproteins. However, exclusive binding to these receptors cannot completely explain the differences in tissue specificity between HHV-6A and -6B. For this reason, it has been postulated that additional unknown factors might be required for HHV-6 entry and survival inside the host cell.
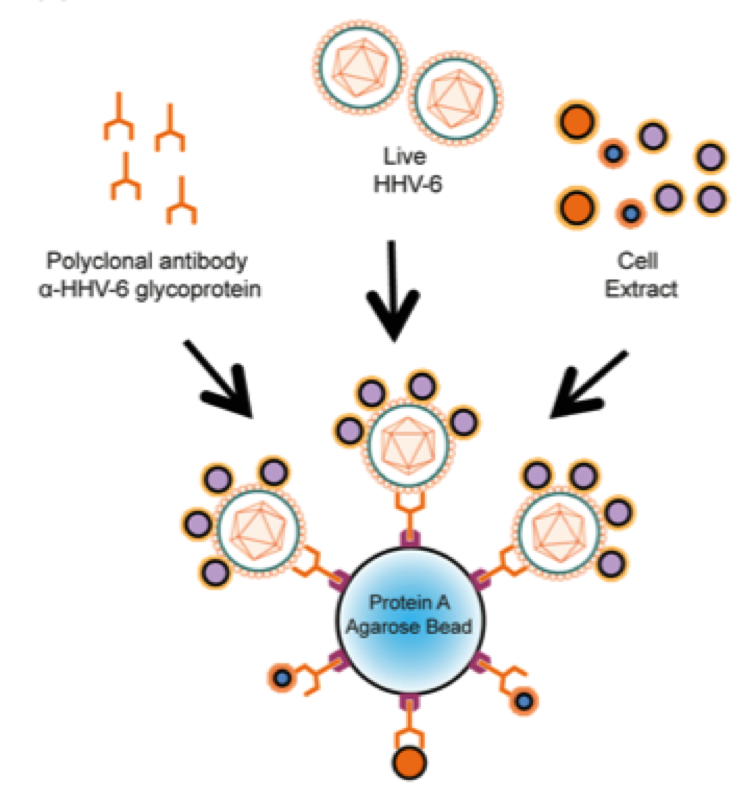
It has been shown that some cells allow HHV-6 productive infection—thus leading to infectious viral progeny formation—while others manage to effectively rid themselves of the infecting virus. To better understand this phenomenon, Prusty and Rudel followed an unbiased proteomics screen and identified human chaperone protein GP96 as a key protein that directly interacts with HHV-6 on the host cell surface and guides it for cellular degradation. “We showed that GP96 acts as a cellular defense against HHV-6 infection and possibly plays a crucial role in deciding the fate of HHV-6 infection,” commented Prusty.
Gp96, also known as a cellular heat shock protein, has been called the immune system’s “Swiss army knife” for its versatility and ability to induce immunity against antigens from the cell from which it came. It plays a critical role in regulating both innate and adaptive immunity.
For more information, read the full paper.
Gp96, the immune system’s “Swiss army knife.
