Advance may aid the study of integration and excision and might ultimately have clinical application.
The primary mechanism by which HHV-6A achieves latency appears to involve integration of its genome into the host telomeres, rather than the formation of a circular episome as is typical for other human herpesviruses. The viral genome also is integrated in the telomeres of every cell in people with inherited chromosomally-integrated HHV-6A (iciHHV-6A). HHV-6 can be excised from its integrated state during reactivation, even in people with iciHHV-6A. The mechanisms of integration and excision remain poorly understood.
Investigators from the laboratories of Louis Flamand at Laval University in Quebec and Benedikt Kaufer at the Freie Universität Berlin used the CRISPR/Cas 9 system to excise the integrated viral genome from both latently infected cells and cells of individuals with iciHHV-6A. Using a polycistronic-tRNA-gRNA (PTG) system the investigators were able to deliver 10 guide RNAs (gRNAs) directed against the noncoding region of the direct repeats (DRs) at the ends of the viral genome with a single plasmid. Efficient excision of the HHV-6A was achieved using constitutive Cas9 expression but also transient Cas9 expression, which was used to minimize potential off-target effects. Removal of the HHV-6A genome was achieved in both latently infected (70-80%) and iciHHV-6A cells (~60%).
Studies of this directed excision revealed that it:
- Did not lead to viral reactivation
- Was not followed by reintegration
- Led to the non-homologous end-joining of the remaining fragments of the right and left DRs
- Maintained the telomeres from which the viral genome had been excised, without a substantial shortening or lengthening
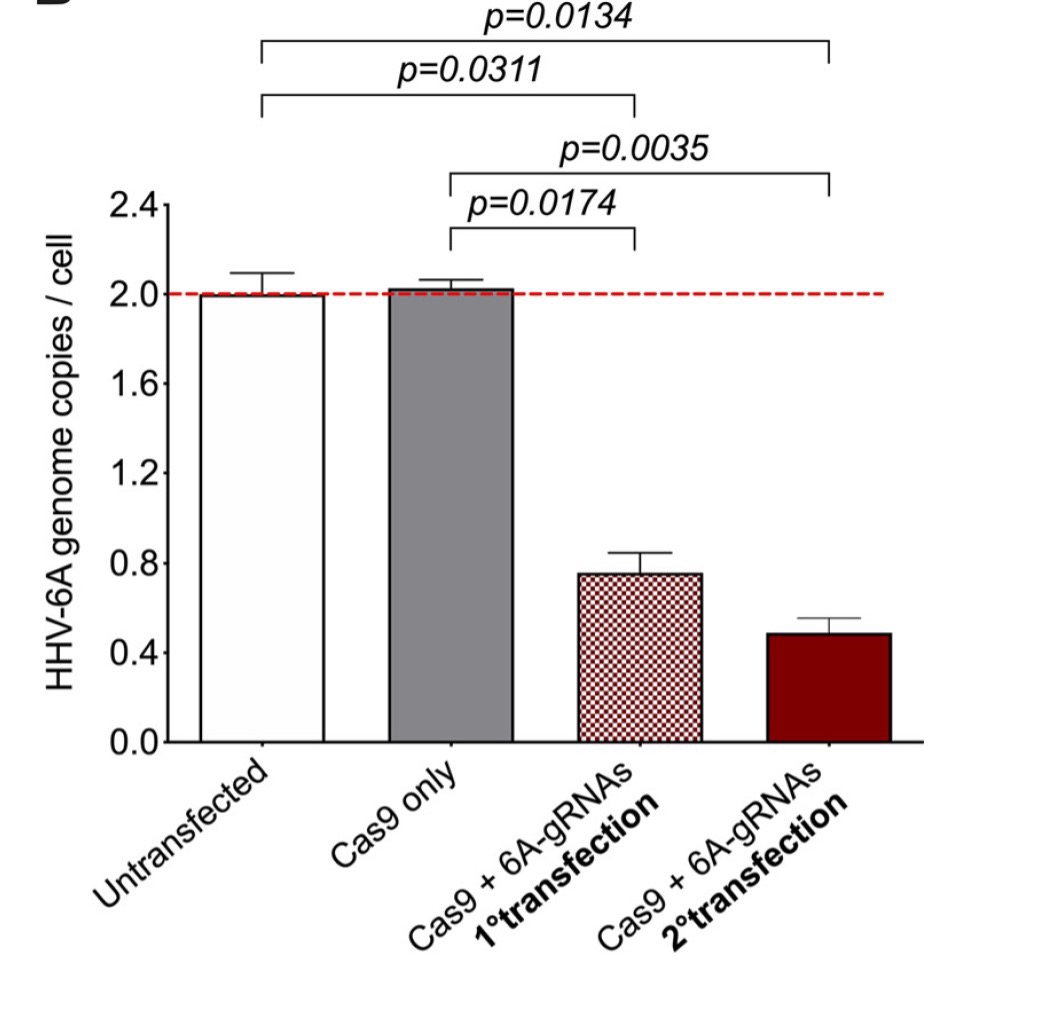
Figure 1 Removal of integrated HHV-6A genome through consecutive transfections. Copy numbers of HHV-6A U86 gene were detected by qPCR.
This technique is a welcome advance, likely to aid the study of the biology of both integration and excision.
Might it someday have clinical applications? The authors speculate that in hematopoietic cell transplantation the integrated virus could be eliminated from latently infected or iciHHV-6 graft cells before they are transplanted. If such a “scrubbing” of virus from graft tissue proved to be achievable, it would remain to be determined whether this reduced post-transplantation complications caused by the virus.
Might the technique ever have value in people with iciHHV-6A? It is hard to envision how the technique might achieve excision of the integrated virus in every cell of a person with iciHHV-6A, and there currently is little evidence that this would have clinical value even if it could be achieved, since no illness has yet been definitively linked to iciHHV-6A. On the other hand, the removal of the integrated virus genome opens new avenues in the research on the endogenous virus genome including its effect on the host cell transcriptome and metabolism.
Read the full article: Aimola 2023