This study helps explain how roseoloviruses maintain persistent infection.
Japanese investigators at Kobe University revealed a novel immune evasion mechanism employed by HHV-6B to manipulate host cell immune responses through its ribonucleotide reductase (RNR) protein. While viral ribonucleotide reductase (RNR) is conserved across betaherpesviruses, it has lost its enzymatic activity in this virus family. Previous research showed that cytomegalovirus (CMV), another betaherpesvirus, uses its RNR to inhibit nuclear factor-kappa B (NF-κB) signaling by interacting with RIPK1. However, the function of enzymatically inactive RNR in roseoloviruses remained unclear until this study
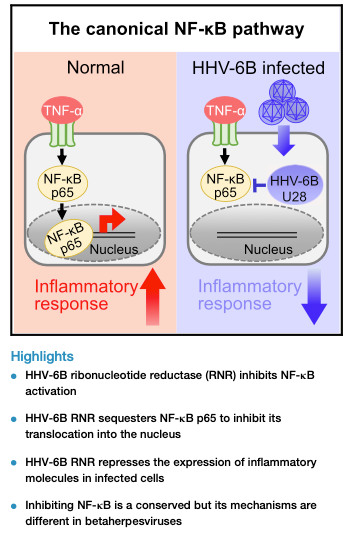
The Kobe University group discovered that RNRs from all three human roseoloviruses (HHV-6A, HHV-6B, and HHV-7) inhibit NF-κB activation. Specifically, the HHV-6B RNR (U28) employs a unique mechanism by directly sequestering the NF-κB subunit p65 in the cytoplasm, preventing its translocation to the nucleus. This sequestration occurs at specific domains within the endoplasmic reticulum.
Furthermore, U28 specifically interacts with p65 but not with other components of the NF-κB pathway (such as p50, RIPK1, or NEMO). This interaction disrupts the binding between p65 and IκBα, leading to the accumulation of inactive p65 in cytoplasmic structures
Through detailed structure-function analyses, the researchers identified that the N-terminal region of HHV-6B U28 is particularly important for p65 sequestration. When this region was swapped with the corresponding region from HCMV UL45, the chimeric protein maintained the ability to inhibit p65 nuclear translocation
When HHV-6B U28 was silenced in infected cells, the expression of inflammatory molecules increased significantly, demonstrating the functional importance of this mechanism during viral infection
RNA-seq analysis revealed that U28 suppresses expression of various inflammatory genes, including those encoding cytokines, chemokines, and antiviral factors.
This study reveals that inhibiting NF-κB is a conserved role of RNR across betaherpesviruses, though the precise mechanisms differ. While HCMV targets RIPK1, HHV-6B directly sequesters p65. This finding enhances our understanding of how HHV-6B evades host immune responses during infection.
The identification of this novel immune evasion strategy provides insights into the pathogenesis of HHV-6B and potentially opens new avenues for therapeutic interventions targeting viral evasion of innate immunity.
The study demonstrates that HHV-6B employs its ribonucleotide reductase U28 to sequester the NF-κB subunit p65 in the cytoplasm, thereby preventing inflammatory gene expression and facilitating viral evasion of innate immune responses. This mechanism represents a significant adaptation that distinguishes roseoloviruses from CMV.
Read the full paper: Hirai 2025