
Mark Prichard, PhD, Professor, Director, Virology Laboratory, University of Alabama at Birmingham
A team at University of Alabama at Birmingham, led by Mark Prichard, PhD, has developed a new method to assess antiviral activity against all DNA viruses using an automated format and qPCR to measure the accumulation of viral DNA. Of the FDA approved drugs, foscarnet showed the highest selectivity index for HHV-6B. Pipeline drugs filociclovir (cyclopropavir) and CMX-001 (brincidofovir) show promise with relatively low EC50 values. However, it is unknown how well those drugs are able pass the blood brain barrier.
Comparing the antiviral properties of various drugs in herpesviruses has led to widely divergent results, in part because of different cell types used and various methods used to assess the replication of the virus which have included: a) expression of viral antigens, or b) accumulation of by viral DNA by methods of DNA hybridization, in situ hybridization, or qPCR. Prichard and colleagues used 384-well plates and utilized qPCR as an endpoint to measure accumulated DNA. For cytotoxicity, they used CellTiter-Glo, a compound that causes ATP in viable cells to generate a luminescent signal.
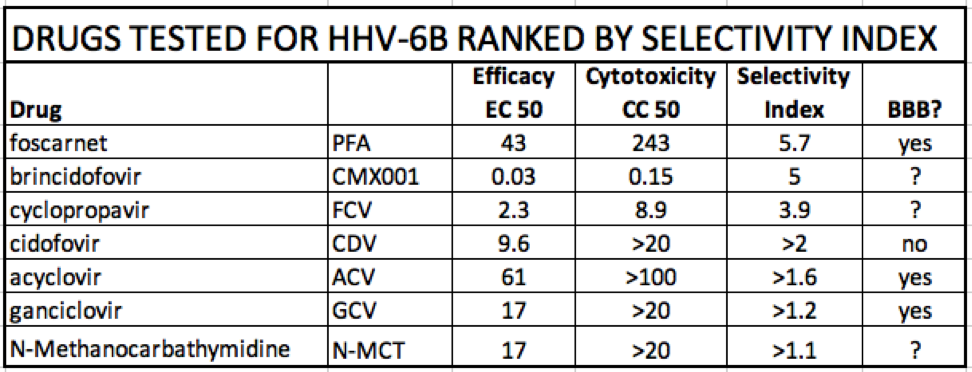 The authors charted the results with the EC and CC 50 values against 5 viruses including HHV-6. In the chart, the selectivity index represents the distance between the average EC50 and CC50 values. The selectivity index for HHV-6B was highest for foscarnet (PFA) with cyclopropavir (FCV) and brincidofovir (CMX001) also showing efficacy. They did not evaluate HHV-6A.
The authors charted the results with the EC and CC 50 values against 5 viruses including HHV-6. In the chart, the selectivity index represents the distance between the average EC50 and CC50 values. The selectivity index for HHV-6B was highest for foscarnet (PFA) with cyclopropavir (FCV) and brincidofovir (CMX001) also showing efficacy. They did not evaluate HHV-6A.
Foscarnet and cidofovir are off patent, and cidofovir is not used for HHV-6 encephalitis because of presumed poor penetration to the brain. IV brincidofovir is currently under development for adenovirus, CMV BK virus and other viruses (including HHV-6) by Chimerix. Although the oral form had very poor organ penetration, the IV form has better penetration to the brain and organs. Cyclopropavir (also known as MBX-400 and filociclovir) is being developed by Microbiotix for CMV.
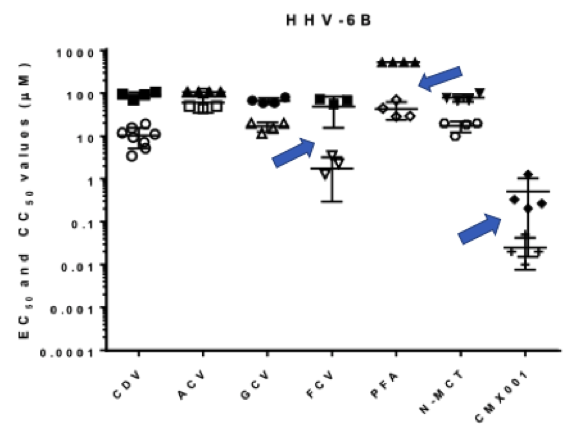
Selectivity index (widest margin between the average toxicity and effective dose) was highest for foscarnet (PFA) followed by filociclovir (FCV). The closed boxes represent toxicity or CC50 values while the open circles represent effective concentration or EC50.
One surprise: a novel compound N-MCT that is being developed for shingles by NN Pharmaceuticals, has about the same efficacy as ganciclovir. The selectivity index for ganciclovir was surprisingly low.
The authors point out that their method allows them to assess antiviral activity with a greatly reduced quantity of compound, and that it works well for compounds that act late in the virus cycle.
Read the full paper: Keith 2018

