Findings provide important insights that may reveal potential targets for antiviral therapy.
Heat shock proteins support viral and cellular proteins that mediate viral entry, capsid transport and viral assembly. The molecular mechanisms by which nascent nucleocapsids of HHV-6A/B acquire a primary envelope during their nuclear export has not been fully defined. With all herpesviruses, the process is mediated by a conserved viral heterodimeric complex: the nuclear egress complex. This complex includes the nuclear matrix protein and the nuclear membrane protein. The nuclear matrix protein also is known to interact with intracellular signaling pathway molecules including NF-κB and IFN-β to affect viral and cellular gene expression. But the mechanisms by which HHV-6A/B-encoded molecules trigger this process remain obscure.
A team from Center for Infectious Diseases, Kobe University Graduate School of Medicine, Kobe, Hyogo, Japan, led by Dr. Yasuko Mori, has addressed this question for HHV-6A. They show that the process by which the nascent nucleocapsids acquire a primary envelope is galvanized by HHV-6A U37, and acts through heat shock transcription factors and proteins.
HEK293T cells were co-transfected with Flag-tagged HHV-6A U37 (Flag-HHV-6A U37) and a series of firefly luciferase reporter plasmids harboring either: the IFN-ß promoter (IFN-ß-luc), the NF-κB-responsive element (NF-κB-luc), the cAMP response element (cAMP-luc), or the heat shock element (HSE-luc).
The experiments showed that:
- Ectopic expression of Flag-HHV-6A U37 did not have any effect on IFN-ß-luc, NF-κB-luc, or cAMP-luc, either in the presence or absence of the individual stimuli.
- However, ectopic expression of Flag-HHV-6A U37 significantly stimulated the activity of HSE-luc. Heat shock increased the HSE-luc activity further in the presence or absence of HHV-6A U37, suggesting that heat shock and HHV-6A U37 synergistically activate the HSE promoter. (See Figure 1)
- HHV-6A U37 first interacts with heat shock transcription factor 1 (HSF1) and induces its phosphorylation at Ser-326, a process dependent on the N-terminal region of HHV-6A U37.
- This induces the accumulation of the molecular chaperone heat shock protein (Hsp) 90.
- Consistent with this, heat shock proteins (HSPs) were up-regulated in HHV-6A-infected cells.
- In addition, pharmacological inhibition of HSF1, Hsp70, or Hsp90 decreased viral protein accumulation and viral replication, indicating the importance of the HSPs in viral replication.
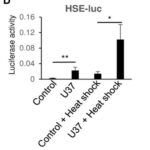
Figure 1. HEK293T cells were co-transfected with HSE-luc together with pRL-CMV as an internal control plasmid, along with either an empty plasmid (control) or plasmid expressing Flag-HHV 6A U37.
Taken together, the results lead the team from Kobe to propose a model in which HHV-6A U37 activates the heat shock response, which is critical in supporting viral gene expression and replication. Although the experiments involved only HHV-6A, the investigators speculate that the same results would be found for HHV-6B, given that the amino acid sequences of HHV-6A U37 and HHV-6B U37 are very similar, with a sequence identity and similarity score of 97% and 100%, respectively.
Read the full article: Huang 2023