Herpesviruses evade host detection through various mechanisms. For example, the glycoprotein gene product, U21, encoded by HHV6A/B and HHV-7 can downregulate MHC I complexes from the cell surface (Glosson 2007).
Whyte et al. (2021) of the Medical College of Wisconsin quantified the expression of N-linked glycoproteins on the cell surface of roseolovirus (HHV6A, 6B, and 7)-infected T cells using mass spectrometry, a reliable and useful way of examining the cell surface proteome during viral infection (Weekes 2014).
They found that HHV-6A and HHV-7 down-regulated the protein tyrosine phosphatase CD45 (CD45) by 87.5%. This protein is expressed on the surface of all nucleated cells of hematopoietic origin and is critical for proper immune function. Various isoforms of CD45 were strongly downregulated by HHV-6A.
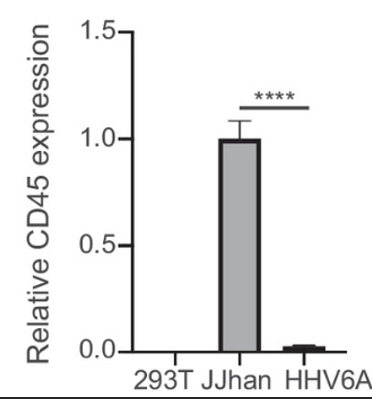
HHV-6A infected cells CD45 mRNA compared to a negative control (293T) and uninfected control (JJhan). qPCR performed in two biological replicates P <0.0001.
CD45 was not internalized in an endosomal compartment, as is found with other viruses; rather, it was depleted by unclear mechanisms. (Proteasomal and lysosomal degradation of CD45 was ruled out). CD45 mRNA was greatly reduced in HHV-6A cells (tested with qRT-PCR), which suggests transcriptional downregulation.
The authors confirmed that TCR stimulation is impaired in HHV-6A infection: levels of metabolic byproducts of TCR signaling were reduced, as compared to noninfected controls. The authors speculate that the downregulation of CD45 may be a means to inhibit activation of HHV-6A-infected T cells: that would prevent activation-induced cell death and thereby create a host cell environment conducive to harboring latent virus.
The functional consequences of CD45 down-regulation in roseolavirus-infected cells remain to be determined.
Read the full article: Whyte 2021