A Chinese group found HHV-6 direct repeat 7 in 48% of glioma tumors. Furthermore, they determined that DR7 overexpression could promote glioma cell migration, invasion and angiogenesis. Expression profiles showed that DR7 created an inflammatory microenvironment that enhanced degradation of the extracellular matrix.
HHV-6A/B infect several types of cells in the brain, which can trigger neurological dysfunction. The pair of viruses are known to cause febrile seizures and encephalitis, as well as epilepsy after encephalitis, and they are still being studied as potential contributors to other conditions, including multiple sclerosis, Alzheimer’s disease, and glioma (Chi 2012, Crawford 2009, Crawford 2009b, Strojnik 2017). Glioma is a type of brain cancer that forms from glial cells, which perform vital functions in the nervous system, including maintenance of myelin sheaths and participation in the immune response. HHV-6 has a unique region that is flanked by 809 kb of termial direct repeats, designated as DR1-DR7. This protein can be detected after 18 hours of viral infection but is not expressed during viral latency.
DR7 has previously been described as an oncoprotein after it was found to transform NIH3T3 cells and produce fibrosarcomas in mice (Kashanchi 1997). Lacroix et al. also found that DR7 protein was present in Reed-Sternberg cells of Hodgkin’s lymphoma and was able to bind and inhibit p53 while enhancing NFκB activity (Lacroix 2010).
The team obtained 27 glioma tissues and 30 normal brain tissues, which were provided by patients who underwent surgery for brain trauma or cerebral hemorrhage. They tested for the HHV-6 protein using a monoclonal antibody provided by the HHV-6 Foundation. Testing revealed the presence of DR7 in 13 (48.15%) gliomas and 5 (16.7%) normal tissues. Additionally, significantly higher expression of DR7 was found among the gliomas compared to the normal samples. Reverse transcriptase PCR confirmed expression of DR7 transcripts in tissues that were positive by IHC. Furthermore, DR7 was detectable in two strains of primary human glioma cells, U87 and U251, by an immunofluorescence assay.
DR7 encoded by HHV-6 promotes glioma development and progression. Cancer Management and Research, Dovepress, 2019
Enhanced cellular proliferation is a key facet of cancer development. In this study, proliferation was measured by cell counting kit-8 testing, and the U87 and U251 cells overexpressing DR7 were found to have a higher proliferation rate compared to blank and negative control-treated cells. The expression of DR7 in U87 cells transfected with DR7-positive lentivirus vectors also increased the colony-forming ability of the cells, as visualized using a soft agar colony formation assay. In the same vein, flow cytometry revealed a higher percentage of cells in the S and G2/M phases of the cell cycle and lower percentage of cells in G0/G1 phase in the DR7-expressing cells compared to the controls. As the S and G2/M phases are indicative of impending or current cellular replication and division, these results suggested that DR7 spurs cellular proliferation by accelerating the cell cycle.
In addition to greater cell replication, metastasis is reliant upon cell migration and invasion, as well as formation of veins to accommodate the high metabolic demands of the cancer. Cell scratch assays revealed faster healing of the glioma cells expressing DR7 compared to controls, and higher numbers of invading cells were seen among DR7-expressing cells than controls using transwell invasion assays. Vascular density measured using CAM assays was also found to be highest for the DR7+ cells.
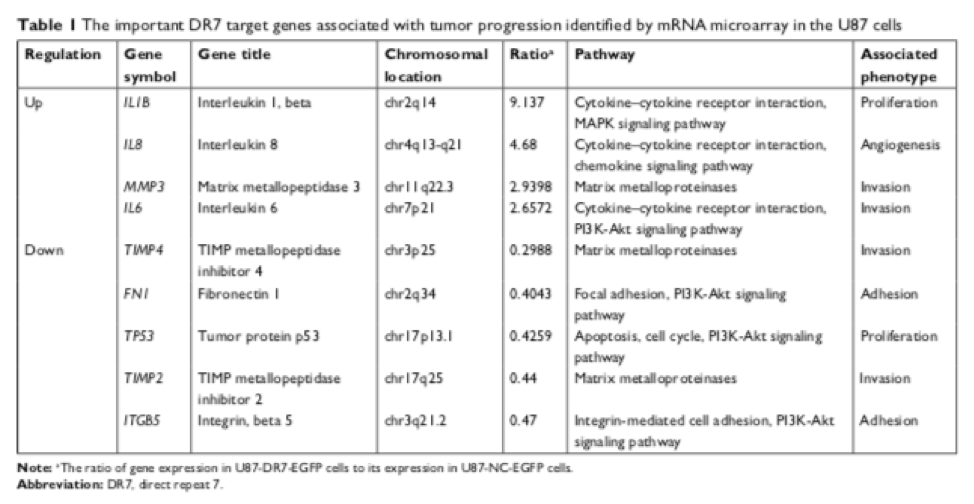
MRNA microarray identified 1,016 upregulated and 778 downregulated genes in the DR7-transfected cells compared to the controls, with several genes known to be important regulators of cell proliferation, migration, invasion, and angiogenesis found to be differentially regulated. Nine of these- IL-1beta, IL-6, IL-8, and MMP-3, which were upregulated, and FN, P53, TIMP-2, TIMP-4, and ITGbeta5, which were downregulated- were selected, and levels of their secreted proteins were measured in DR7+ and control cells using ELISA and Western blot. Translation of these proteins corresponded with the up/downregulation patterns of the mRNA. Together, the results suggested that these genes are involved in the DR7-associated increase in glioma cell proliferation, invasion, migration, and in angiogenesis, perhaps by creating an inflammatory microenvironment, disrupting the NFκB, PI3K-Akt, and JAK-STAT pathways, and degrading extracellular matrix components.
The authors note that while the HHV-6 gene U94/rep has anti-angiogenic functionality, underexpression of U94, overexpression of DR7, or both could still result in increased angiogenesis in HHV-6-infected cells.
One unique case has been reported in which a young child with HHV-6 encephalitis went on to develop a pilocytic astrocytoma, the most common type of pediatric glioma, containing HHV-6 DNA 11 months after the primary infection (Rantala 2000). In another report, a 2-year-old who developed neurological symptoms and hydrocephalus, several months after experiencing a skin rash with irritability and fatigue, was found to have HHV-6A in his cerebrospinal fluid (CSF) and serum. Antiviral treatment improved symptoms temporarily several times, and chemotherapy was initiated after diagnosing the child with primary leptomeningeal oligodendrogliomatosis, a rare malignancy that may stem from heterotopic nests of glial cells. HHV-6A DNA was detected in the tumor tissue and was considered to at least have contributed to the clinical course (Stödberg 2002).
Recent studies have found correlations between HHV-6 activity and other cancers, including pituitary adenoma, ovarian cancer, gastrointestinal cancers, and non-Hodgkin lymphoma. Research into these topics is ongoing.
Find the full paper here: Gu 2019.