Two new reviews conclude that HHV-6 acute necrotizing encephalopathy is tied to a mutation of the RANBP2 gene. RANBP2 is a gene that codes for protein with an essential role in energy metabolism in neuronal cells, and a defect in this gene results in an energy breakdown when the cell is challenged by viral infections (such as with HHV-6, influenza, parainfluenza, varicella) as well as intracellular bacterial infections such as mycoplasma pneumonia (Singh 2015).
HHV-6 encephalopathy usually falls into the category of acute encephalopathy with biphasic seizures and reduced diffusion (AESD), but rarely causes acute necrotizing encephalopathy (ANE) with high CSF protein multi-focal symmetrical brain lesions (Hoshino 2012).
Infection-induced acute encephalopathy 3 (IIAE3) refers to the susceptibility to recurrent acute necrotizing encephalopathy IIAE3 is diagnosed based on the presence of a mutation of RANBP2, which is inherited in an autosomal dominant fashion. The first episode of IIAE3 typically affects individuals younger than six years of age, predominantly those between nine months and two years of age. However, teenagers and adults may also experience first episodes of IIAE3, and half of the patients with IIAE3 experience at least one repeat episode. Although additional episodes typically take place during childhood, they may also occur in teenagers and adults.
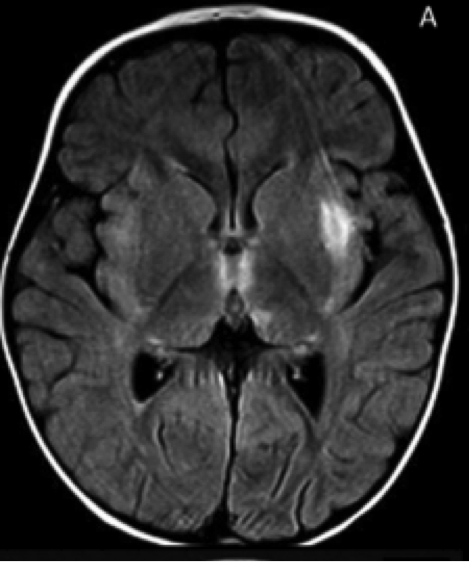
IIAE3 is suspected in individuals with no previous developmental or neurological defects, and lethargy that progresses to coma and seizures. Upon admission to the hospital, individuals with IIAE3 typically demonstrate and bilateral thalamic involvement T2- and FLAIR-weighted hyperintensities in multiple brain regions. One out of three individuals die during the acute phase of encephalopathy. Survivors exhibit loss of developmental skills, such as walking and speaking, which may be regained over time. Fifty percent of survivors recover without discernable mental impairment, while the remaining survivors suffer from permanent neurologic damage. Patients who undergo recurring episodes experience greater levels of cellular damage.
Methods of treatment include administration of corticosteroids, IVIG, plasmapheresis, and TNF-a antagonists. Early identification of a RANBP2 pathogenic variant via molecular genetic testing, yearly influenza vaccinations and routine vaccinations, and attention to behavioral changes during febrile episodes may all assist in prevention and early intervention in patients experiencing ANE.
For more information read the full text: Neilson 2014 and Singh 2015.
Mutation of the RANBP2 gene.
