European guidelines recommend treating HHV-6 disease with either foscarnet or ganciclovir, in contrast to the Japanese guidelines that recommend foscarnet as first line treatment due to a lower mortality rate.
The new guidelines were developed by specialists in the field of infectious disease, oncology and transplantation for the European Conference on Infections in Leukemia (ECIL) for diagnosing, preventing, and treating HHV-6 disease. Japanese guidelines were published last year, and an English version is pending.
Among the recommendations and observations made by the European group:
- To accurately identify HHV-6 infection, distinctions between HHV-6A, HHV-6B, and chromosomally integrated HHV-6 (ciHHV-6) must be ruled out.
- Finding HHV-6A in a transplant patient is suggestive of ciHHV-6.
- ciHHV-6 can be differentiated from HHV-6B by higher levels of viral DNA in whole blood samples and the detection of viral DNA in hair samples.
- If a donor has ciHHV-6, viral DNA in the recipient will increase with leukocyte engraftment
- If ciHHV-6 can be ruled out and HHV-6B is suspected, antiviral therapy is well advised.
- The 80.5% survival rate of allogenic HSCT drops to 58.3% in patients that develop encephalitis, and of those who survive 57% suffer lasting neurological sequelae.
- Post-HSCT encephalitis in children may cause the development of a novel syndrome characterized by generalized epilepsy, cognitive regression, and delayed intellectual development.
- Typically, the virus exerts its effects through reactivation, rather than primary infection, and often presents as post-transplant limbic encephalitis (PALE) with symptoms of headaches, delirium, and neurocognitive decline.
- An increase in CSF cell count may be observed but is usually mild.
- Quantitative PCR that distinguishes between HHV-6A and B is recommended to diagnose the infection and repeat testing in the same patient should use the same method of DNA extraction and analysis.
- The diagnosis of HHV-6 encephalitis should be defined by the presence of HHV-6 DNA in CSF and symptoms of acute-onset altered mental status, short term memory loss, or seizures.
- Myelosuppression, umbilical cord blood transplants, T-cell depleted allografts, mismatched or unrelated donors, and treatment of acute GVHD with glucocorticoids all increase the chances of developing HHV-6 encephalitis. However, it is difficult to use these predictive factors to catch HHV-6 encephalitis early.
- Pre-screening is not recommended because the disease usually onsets as the virus reactivates, and prophylactic and preemptive treatments fail to significantly decrease instances of HHV-6 encephalitis.
- The recommended course of action is to treat the active infection therapeutically with foscarnet and/or ganciclovir.
- Foscarnet monotherapy appears to exhibit lower rates of overall mortality than ganciclovir monotherapy, but definitive conclusions on the superior efficacy of one or the other are difficult to draw due to small sample sizes of different patients on different regimens.
- Both medications have severe side effects, but an effective alternative may soon be provided by a new immunotherapy using virus-specific T-cells that does not appear to share the nephrotoxic and myelosuppressive risks associated with foscarnet and ganciclovir respectively.
- In addition to encephalitis, HHV-6 has been implicated in post-HSCT acute GVDH and pneumonitis, the leading cause of post-HSCT morbidity.
- The methods for documenting cases of end-organ HHV-6 infection need improvement in order to properly assess the role of HHV-6 in these complications.
- Tissue from organs suspected to be infected with HHV-6 should be tested by culture, immunochemistry, in situ hybridization, or by reverse transcription PCR for mRNA.
- Simply testing for HHV-6 DNA in the tissue via qualitative PCR is not sufficient to document cases of HHV-6 infection.
Routine HHV-6 testing was not suggested for cord blood transplantat patients, in spite of an 8% rate of HHV-6 encephalitis in that population.
Read the full paper: Ward 2019
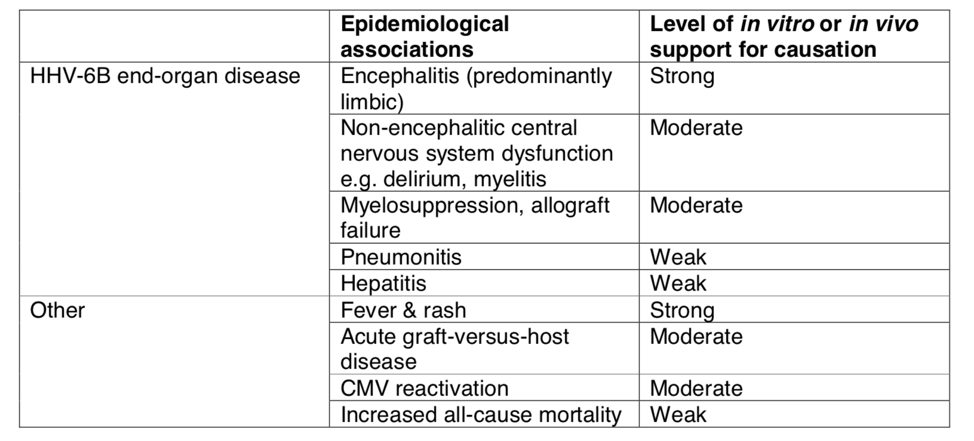
HHV-6B disease associations as described by the European working group.

