HHV-6B directly infects thymocytes, presumably affecting thymopoeisis. A review in Bone Marrow Transplantation explores the intriguing relationship between HHV-6B, T-cell reconstitution and aGVHD after allogenic hematopoietic cell transplantation.
T-cell reconstitution after HCT has two phases: 1) an immediate, thymus-independent phase, in which T-cell reconstitution takes place primarily in the periphery, and 2) a late, prolonged, multi-step, thymus-dependent phase that can take up to 18 months after HCT. A group of HHV-6 experts led by NIAID senior investigator Paolo Lusso, MD, PhD proposed several mechanisms whereby HHV-6B could affect both early and late T-cell reconstitution.
Direct infection of peripheral T cells by HHV-6B during the early reconstitution phase could be a cause, as well as a consequence, of selective CD4+ lymphopenia. de Pagter et al. reported that HHV-6-specific responses of CD8+ cells, but not CD4+ cells, were significantly increased in patients who reactivated HHV-6 compared to those that did not reactivate HHV-6 (de Pagter 2012). A similar but older study also reported stronger CD4+ proliferative responses in patients who did not reactivate HHV-6 compared to those that did (Wang 1999). Given that HHV-6B is CD4+ lymphotropic, these are peculiar findings. However, Phan et al. proposed that CD4+ cells, including CD4+ CD25+ FOXP3+ regulatory T cells, could have been depleted by HHV-6B infection, resulting in dysregulated proliferation of potentially alloreactive CD8+ cells, leading to aGVHD. The authors of the review also summarized a series of case reports and clinical studies describing HHV-6-associated selective CD4+ lymphopenia.
Next, the authors focused on how HHV-6B infection could be affecting long-term CD4+ reconstitution (also previously covered here). Briefly, Admiraal et al. showed that HHV-6 infection was associated with worse CD4+ reconstitution and increased risk of developing grade 2-4 aGVHD. In the follow-up study by de Koning et al., HHV-6 was associated with long-term CD4+ depletion, an effect that was ameliorated by antivirals effective against HHV-6 (ganciclovir, foscarnet, and cidofovir). This late phase of T-cell reconstitution is dependent on the thymus and previous studies have shown that HHV-6 can productively infect and deplete human thymic tissue implanted in mice. A more recent study demonstrated that MRV (a murine analog of HHV-6) causes severe thymic necrosis in neonatal mice. Intriguingly, CD134, the receptor used by HHV-6B to gain cell entry, can be expressed on thymocytes and has also been shown to antagonize regulatory T cell function. It is likely that the effects of HHV-6B and CD134, a receptor expressed on activated CD4+ cells, are highly correlated, particularly in the HCT setting.
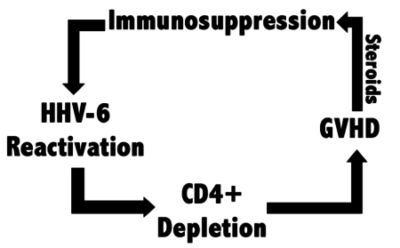 The authors also speculate on how these processes might lead to downstream aGVHD and propose that HHV-6B may be associated with steroid-refractory aGVHD. HHV-6B reactivation has been associated with delayed immune reconstitution after HCT as well as acute graft-versus-host disease (aGVHD) (Phan 2018).
The authors also speculate on how these processes might lead to downstream aGVHD and propose that HHV-6B may be associated with steroid-refractory aGVHD. HHV-6B reactivation has been associated with delayed immune reconstitution after HCT as well as acute graft-versus-host disease (aGVHD) (Phan 2018).
Steroids have been shown to disproportionately reactivate HHV-6. They hypothesized that aGVHD (possibly secondary to HHV-6B-induced CD4+ depletion) is often treated by steroids, which further exacerbates T-cell depletion and aGVHD. It is possible that HHV-6 may not be directly causing aGVHD, but rather HHV-6 may be a bystander that significantly exacerbates the disease, similar to estrogen in ER+ breast cancer.
Read the full paper: Phan 2018

