A higher prevalence of inherited ciHHV-6 also found in patients vs donors.
In a recent analysis of over 8,500 subjects – representing over 4,000 unique stem cell transplant donor-recipient pairs – investigators at Fred Hutchinson Cancer Center led by Joshua Hill and Michael Boeckh have found that acute graft-versus-host disease (aGVHD) occurs twice as often in transplants when either donor or recipient is found to have inherited ciHHV-6. In addition, transplant recipients with iciHHV-6 were three times more likely to develop high level cytomegalovirus (CMV) viremia in their study.
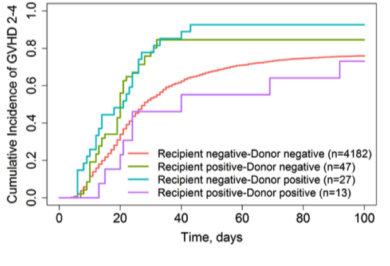
Cumulative Incidence of acute GVHD according to iciHHV-6 status (Source: Blood, American Society of Hematology)
HHV-6A and HHV-6B have a unique ability to integrate into human chromosomes as a form of latency, and when viral integration occurs in a germline cell, the offspring are born with a full copy of the HHV-6 genome in every nucleated cell. Although this was originally thought to be a “dead end” form of latency which occurs asymptomatically in approximately 1% of the general worldwide population, integrated HHV-6 is now known to generate gene expression and reactivate in response to certain drugs, including steroids, as well as in patients who are immunocompromised (Endo 2014).
The present study also found that transplant patients were significantly more likely to have iciHHV-6 than donors, as 0.9% of 4,319 donors had the inherited genome compared to 1.4% of cancer patients receiving a transplant – a finding which suggests iciHHV-6 status may impact the likelihood of developing cancer.
While early HHV-6 reactivation post-transplantation has been found to increase the risk of aGVHD in multiple studies (Cirrone 2016, Verhoeven 2015, Gotoh 2014, De Pagter 2013, Zerr 2012), and several studies have shown that HHV-6B reactivation enhances the risk of CMV reactivation (by as much as 15X in one study (Crocchiolo 2016)), this is the first study to demonstrate that inherited ciHHV-6 is also associated with aGVHD and a higher risk of CMV reactivation.
In multivariable Cox proportional hazards models, the adjusted hazard ratio for aGVHD in patients with iciHHV-6 or with iciHHV-6 donor cells was 1.7 and 1.9 respectively (p=0.004 and p<0.001). Patients with iciHHV-6 had were more likely to develop both low level cytomegalovirus viremia as well as high level infections (HR of 1.7 and 3.1; p= 0.001- 0.040). HHV-6B was found in 71% of ciHHV-6+ individuals compared to 29% with HHV-6A. The adverse consequences of iciHHV-6 status were similar whether the donor or recipient was positive for iciHHV-6. A small number of the donor-recipient pairs were both positive for iciHHV-6, but these were mostly related family members, making a comparison to unrelated pairs more difficult.
The authors of the study recommend that screening for inherited ciHHV-6 could be considered to guide donor selection, risk stratification, and treatment strategies after transplantation. “Further study is required to replicate these findings,” adds Dr. Joshua Hill, primary author of the study, “and to understand the direct or indirect mechanisms by which inherited ciHHV-6 may lead to the associations demonstrated in this study.”
In a recent review published in Blood, Karolinska Institute Professor Per Ljungman – a world-renowned HCT expert – considers these findings and the newly developing story of ciHHV-6 reactivation, as well what it might mean for transplant patients and centers moving forward. In his review, Pr. Ljungman considers several possible mechanisms of ciHHV-6 reactivation which could ultimately result in clinical disease. Although he is hesitant to recommend the adoption of routine ciHHV-6 screening in all patients at this time, he urges the international transplant community to collaborate on multicenter studies focused on furthering our understanding of how to manage ciHHV-6 reactivation in the setting of transplantation.
Read the full paper: Hill 2017b